
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Flow cytometry is a powerful technique for measuring and analyzing the physical characteristics of single particles in solution as they travel past a beam of light. Properties such as relative size and fluorescence can be measured, and fluorescently tagged antibodies enable cells to be interrogated for multiple proteins and molecular dynamics. Isotype controls are used for experiment validation and analysis of results.
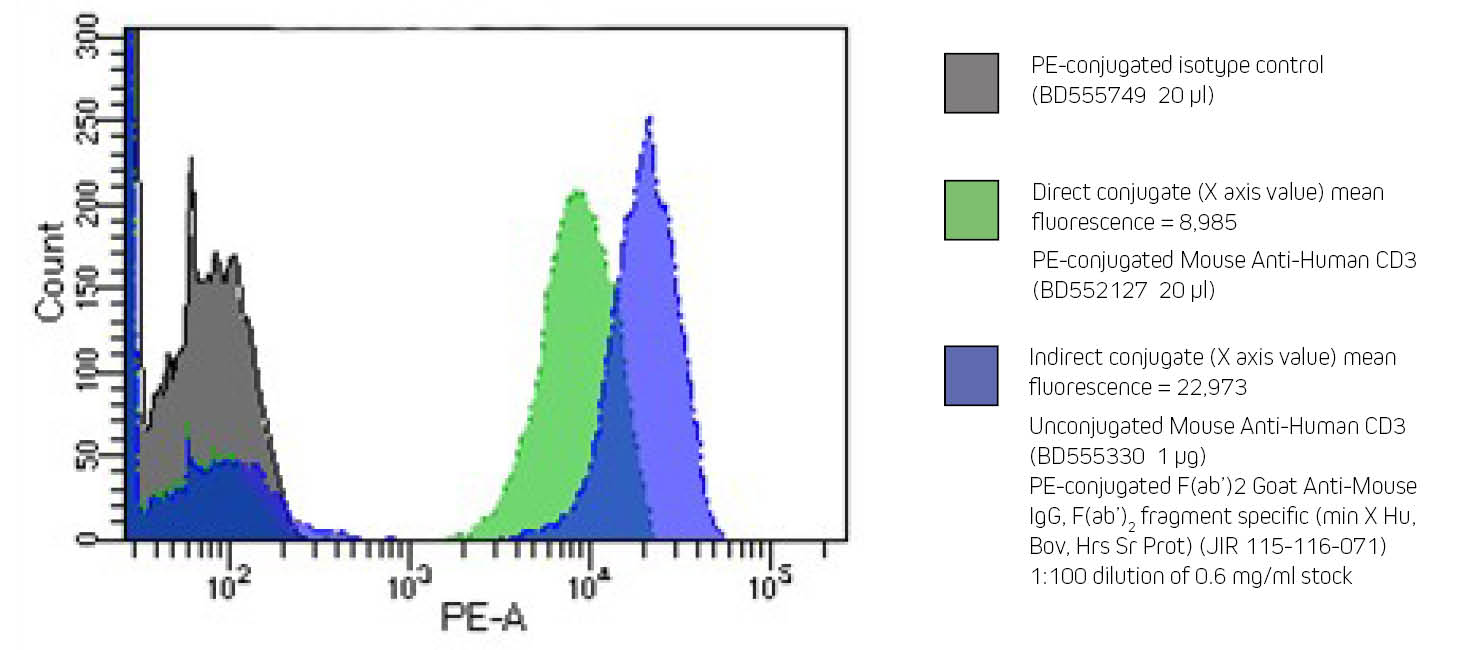
Flow cytometry can be performed directly, using conjugated primary antibodies, or indirectly, using a conjugated secondary antibody to bind an unconjugated primary. Indirect flow cytometry allows the choice of a wide range of probe molecules, enabling the user to match the desired probe with any primary antibody. Secondary antibody conjugates can improve a flow cytometry experiment by preserving the active site of the primary antibody, and by signal amplification (see Figure 1 B).
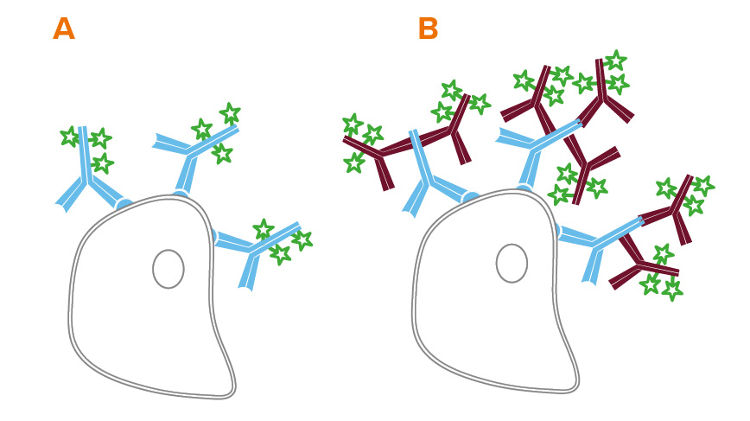
The format of secondary antibody can impact the success of an experiment. In addition to whole molecule IgG, Jackson ImmunoResearch offers fragments of secondary antibodies.
F(ab')2 fragments are generated by proteolysis of the whole IgG to yield a divalent fragment containing two Fab arms and no Fc domain. When used to stain tissue or cells, the F(ab')2 secondary antibodies can help to avoid background caused by off-target binding. The absence of an Fc region prevents F(ab')2 antibodies from being captured by Fc receptors expressed on cell surfaces. Please note that if a primary antibody is trapped by an Fc receptor, the F(ab')2 secondary antibody will detect the off-target binding, so blocking is critical.
FabuLight™ secondary antibodies are created by papain digestion of IgG, followed by removal of Fc fragments. These monovalent Fab fragments are specific for the Fc region of primary antibodies, so they don't interact with the primary's antigen-binding region. Conjugated FabuLights are convenient for labeling primary antibodies prior to incubation with an experimental sample, saving incubation and wash steps. Like F(ab')2 fragments, these Fab fragments can minimize background staining due to Fc receptor binding.
The format of JIR's AffiniPure-VHH™ secondary antibodies makes them an excellent choice for flow cytometry. Being smaller than conventional antibodies and fab fragments at 15kDa, they can decorate the primary antibody with high efficacy. In addition, they lack an Fc domain, limiting potential effector activation and internalization. Finally, their polyclonality ensures heterogeneous binding of the primary antibody, maximizing detection across the whole molecule to ensure optimal signal.
The choice of fluorescent dye conjugate depends on a number of experimental variables.
Fluorescent dyes from UV to far red can be used for flow cytometry, depending on instrument capabilities. Jackson ImmunoResearch offers a range of fluorescent dye conjugates spanning the spectrum, making it possible to design a flow cytometry dye panel that accommodates instrument capabilities and recombinant proteins incorporated in the experiment. These secondary antibody conjugates can be found listed in the tables of Whole IgG and F(ab')2 Fragments.
Jackson ImmunoResearch offers three large, bright fluorescent proteins (R-PE, APC, and PerCP) conjugated to a selection of highly adsorbed secondary antibodies, streptavidin, and purified immunoglobulin controls. The conjugates are excellent choices for surface labeling, but their size may preclude their use as intracellular probes.
Jackson ImmunoResearch offers Biotin-SP-conjugated secondary antibodies in both whole IgG and F(ab')2 format. Biotin-SP conjugates require the use of fluorescently labeled streptavidin for visualization.
Controls are essential to validate an experiment and interpret results. ChromPure™ proteins are purified from the serum of non-immunized animals and are appropriate experimental controls. An isotype control is a negative control which estimates the non-specific binding of an antibody. Isotype controls are antibodies which match the host species and class of antibodies used in the experiment but are not directed against the antigen of interest. An isotype control should be conjugated with the same reporter molecule as the specific antibody.
An isotype control for direct immunofluorescence will be conjugated to the same fluorophore as the primary antibody. For polyclonal primary antibodies (e.g. rabbit or goat primaries), conjugated ChromPure purified proteins are good experimental controls. Conjugated ChromPure proteins can also be used as controls for monoclonal primaries, though it may be preferable to use a control that is the same subclass as the monoclonal.
Indirect immunofluorescence may require isotype controls for both the primary and secondary antibodies
The dilutions suggested in the following table are presented as ranges because the optimal dilution is a function of many factors, such as antigen density, permeability, etc. The optimal working dilution should be determined empirically for each application.
| Product | Dilution |
|---|---|
| Unconjugated whole IgG and F(ab')2 secondary antibodies | 10 - 20 µg / ml |
| Unconjugated Fab secondary antibodies | 20 - 40 µg / ml |
| All Alexa Fluor®, DyLight, and Cy3 Conjugates [IgG, F(ab')2, Fab] | 1:100 - 1:800 |
| Brilliant Violet™ Conjugates [IgG] | 1:50 - 1:200 |
| AMCA, Cy2, FITC, TRITC, RRX, and Texas Red Conjugates [IgG, F(ab')2, Fab] | 1:50 - 1:200 |
| Cy5 Conjugates [IgG] | 1:100 - 1:400 |
| Phycoerythrin and Allophycocyanin Conjugates [IgG, F(ab')2, Streptavidin] | 1:50 - 1:200 |
| PerCP Conjugates [IgG, F(ab')2, Streptavidin] | 1:25 - 1:100 |
| Biotin-SP Conjugates [IgG, F(ab')2, Fab] | 1:200 - 1:1,000 |
| All Alexa Fluor®-, DyLight 405-, and Cy3-Conjugated Streptavidin | 0.5 - 2 µg / ml |
| Cy5-Conjugated Streptavidin | 1 - 4 µg / ml |
| All Other Fluorophore-Conjugated Streptavidin | 2 - 5 µg / ml |