Autofluorescence can present challenges for techniques such as immunohistochemistry (IHC), immunocytochemistry (ICC), and flow cytometry, where it may reduce assay sensitivity by increasing background or may even mask the specific signal altogether. This article looks at some common causes of autofluorescence and suggests ways to minimize its impact.
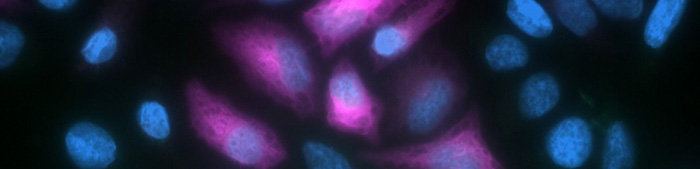
What is autofluorescence?
Autofluorescence is a term used to describe the background fluorescence observed in biological samples that does not result from staining with a specific fluorescent probe. It is most prominent in the green channel, where it can interfere with the signal from antibody-conjugated fluorophores such as Alexa Fluor® 488 and fluorescein isothiocyanate (FITC), potentially obscuring the detection of analytes with low abundance. In addition, autofluorescence can hamper the detection of green fluorescent protein (GFP), which is widely used as a fusion tag and as a quantitative reporter for gene expression.
Common sources of autofluorescence
Many endogenous sample components can autofluoresce. The following are some of the best-known examples:
- Collagen – the main component of the extracellular matrix (ECM)
- Riboflavin (vitamin B2) – the precursor to flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD), which are coenzymes with essential roles in metabolism
- NADH – the reduced form of nicotinamide adenine dinucleotide (NAD), a coenzyme primarily involved in the production of ATP through oxidative phosphorylation in mitochondria
- Elastin – an ECM component responsible for the extensibility and elastic recoil of tissues, including blood vessels, lungs, and skin
- Lipofuscin – a pigmented byproduct of intracellular catabolism that accumulates progressively over time in the lysosomes and cytosol of post-mitotic cells such as myocytes and neurons
- Heme – an iron-protoporphyrin complex found in hemoproteins including hemoglobin, myoglobin, and cytochrome P450
- Aromatic amino acids – phenylalanine, tryptophan, and tyrosine
- Melanin – a pigment produced by melanocytes and found in the hair, skin, and iris, which protects against ultraviolet (UV) rays and reactive oxygen species (ROS)
- Chlorophyll – a green pigment, found in plants and cyanobacteria, which absorbs light to provide energy for photosynthesis
- Lignin – a complex heteropolymer found in plant cell walls
In addition, autofluorescence can come from common laboratory reagents and consumables. These include the following:
- Plastic microplates and cell culture flasks – these should ideally be substituted with glass-bottomed or nonfluorescent polymer containers for fluorescence imaging studies
- Media supplements – additives such as Fetal Bovine Serum (FBS) and Phenol Red should be avoided when performing live-cell imaging studies
- Aldehyde fixatives – formaldehyde, paraformaldehyde, and glutaraldehyde can yield autofluorescence by reacting with amine groups to form Schiff’s bases. To mitigate against this effect, samples can be fixed with an organic solvent such as ice-cold methanol. Alternatively, aldehyde-fixed samples can be treated with sodium borohydride, which is known to neutralize Schiff’s bases by reducing amine-aldehyde compounds into non-fluorescent salts.
How to evaluate sample autofluorescence
To determine the level of autofluorescence present in a sample, researchers are advised to run an unstained control and observe the sample using available filter sets. This simply involves performing the experiment as per usual, but omitting the fluorescently-labeled reagent. If autofluorescence is detected, the experimental protocol may require optimization.
Ways to manage autofluorescence
Besides the methods previously described, many other approaches have been developed to reduce autofluorescence. Choosing which to use will depend on factors including the sample type, fixation method, and application being performed, but the following are some tried and tested strategies:
- Select fluorophores that are spectrally distinct from any observed autofluorescence; for example, consider using a Near-Infrared (NIR) Fluorescent Conjugate if autofluorescence is detected in the green channel
- Titrate fluorescent reagents to maximize the signal-to-background ratio
- If you’re using a GFP tag and finding background signal in the GFP channel you can try using an Anti-GFP Antibody Conjugate to switch detection away from the green channel
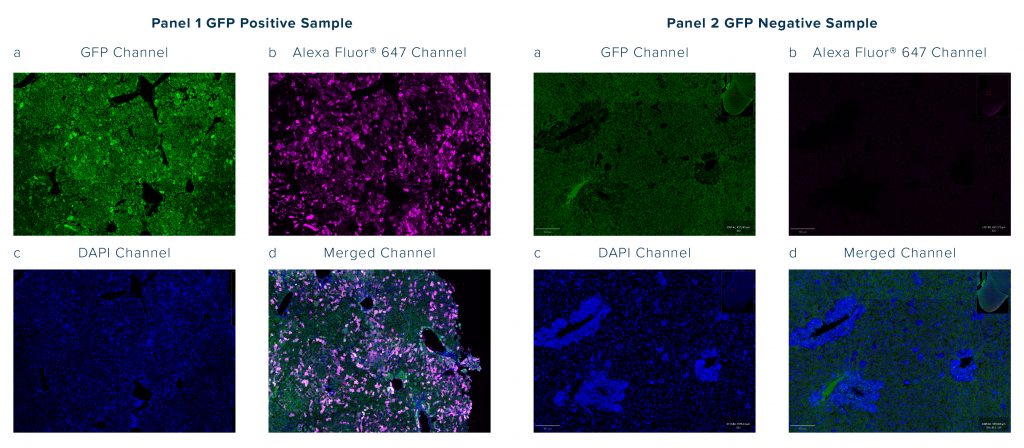
- For flow cytometry experiments, use a viability dye to gate out dead cells and debris that can exhibit high levels of autofluorescence.
- Remove red blood cells from tissue samples by perfusing with Phosphate Buffered Saline (not applicable to archived or post-mortem tissue), or from whole blood by performing a lysis step followed by washing
- Consider performing an autofluorescence-reducing treatment; for example, photobleaching samples prior to incubation with fluorescently-labeled antibodies, incubating samples overnight in 5% H2O2, or quenching autofluorescence with Sudan black B.1,2,3
- Think about switching from traditional flow cytometry to spectral flow cytometry, or from conventional fluorescence microscopy to fluorescence lifetime imaging microscopy (FLIM), to allow for separating autofluorescence from immunofluorescence signals4,5
- Always include relevant controls to ensure results are interpreted correctly
Learn more about Anti-GFP Antibody Conjugates from Jackson ImmunoResearch in this Technical Note, which includes links to application-specific case studies
References
- Sun Y, Ip P, Chakrabartty A. Simple Elimination of Background Fluorescence in Formalin-Fixed Human Brain Tissue for Immunofluorescence Microscopy. J Vis Exp. 2017;(127):56188. doi:10.3791/56188
- Willows JW, Blaszkiewicz M, Townsend KL. A clearing-free protocol for imaging intact whole adipose tissue innervation in mice. STAR Protoc. 2022;3(1):101109. doi:10.1016/j.xpro.2021.101109
- Sun Y, Yu H, Zheng D, et al. Sudan black B reduces autofluorescence in murine renal tissue. Arch Pathol Lab Med. 2011;135(10):1335-1342. doi:10.5858/arpa.2010-0549-OA
- Roet JEG, Mikula AM, de Kok M, et al. Unbiased method for spectral analysis of cells with great diversity of autofluorescence spectra. Cytometry A. 2024;105(8):595-606. doi:10.1002/cyto.a.24856
- Hwang W, McPartland T, Jeong S, Evans CL. A robust method for autofluorescence-free immunofluorescence using high-speed fluorescence lifetime imaging microscopy. Sci Rep. 2025;15(1):5503. doi:10.1038/s41598-025-89142-6



