Visualization of the target proteins is the object of a western blot. There are a number of different detection methods which will require choice of the correct secondary antibody conjugate. They offer different advantages. Read part 8 to find out more.

Overview
- Colorimetric Detection
- Chemiluminescent Detection
- Substrates for Chemiluminescent Detection
- Enhanced Chemiluminescence (ECL)
- Charged-Coupled Device (CCD) Camera
- Film Capture
- Detecting Multiple Proteins with Chemiluminescent Detection
- Fluorescent Detection
- Detecting Multiple Target Proteins on the Same Blot – Multiplex Detection
- Dye Selection
- Lenses and Data Collection
- Multichannel Imagers
- Autofluorescence
- Far-Red and Infrared Dyes
- References
Target proteins can be visualized using a variety of detection methods. Although radioactive and colorimetric detection has been used historically, chemiluminescent and fluorescent detection have become increasingly popular.
Colorimetric and chemiluminescent detection utilize reporter enzyme conjugates. The enzymes catalyze reactions with their substrates to generate products that can be detected. Colorimetric detection enables quick and straightforward visualization of the protein of interest without the need for expensive instruments.
Colorimetric substrates yield a colored product that can be viewed by the eye or quantified using a camera and detection software. Chemiluminescent substrates emit light, which may be visualized using film or a CCD camera. Fluorescent western blotting uses a fluorescent dye molecule to provide a signal which is captured using an imager and may be used to facilitate the detection of multiple target proteins simultaneously.
Colorimetric Detection
Colorimetric detection offers fast results and is simple to perform, making it an attractive option for many researchers. The membrane is incubated with a reporter enzyme-conjugated secondary antibody, either Alkaline Phosphatase (AP) or Horseradish Peroxidase (HRP), after which a colorimetric substrate is added to the blot. The substrates are converted to colored, insoluble products, which appear as colored spots or “bands” where the target molecule: antibody complex are captured on the membrane.
The reaction can take a few minutes for a precipitate to develop. The bands are developed until the desired color intensity is reached before a stop solution is added to “quench” the reaction and prevent oversaturation, which may prevent result interpretation. The membrane is then “set” and can be analyzed by eye without imaging equipment. This process is shown in Figure 1.
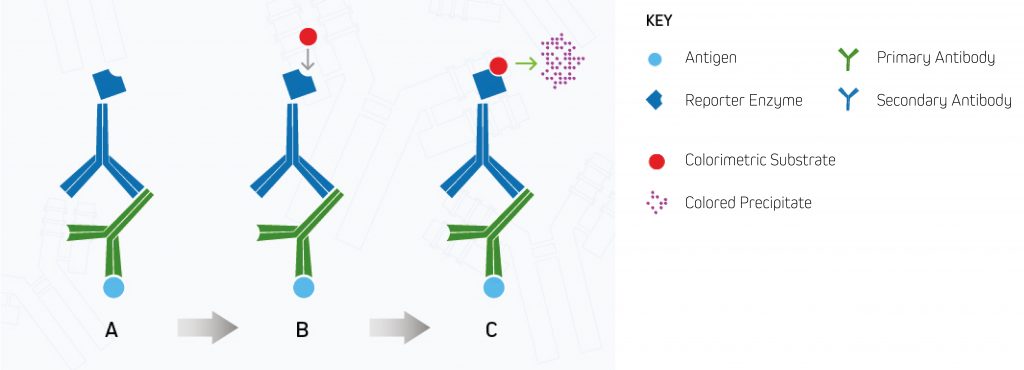
A. Enzyme-conjugated secondary antibody binds the primary antibody at the protein of interest.
B. The substrate is added to the antibody-antigen complex.
C. The enzyme reacts with the substrate, forming a detectable colored product.
Colorimetric Substrates for Alkaline Phosphatase
Nitro blue tetrazolium (NBT) and 5-Bromo-4-chloro-3-indolyl phosphate (BCIP) are two AP substrates used in combination with colorimetric detection to give a blue-purple precipitate.
Colorimetric Substrates for Horseradish Peroxidase
Commonly used substrates for HRP include 3,3′, 5,5′-tetramethylbenzidine (TMB), 3,3-diaminobenzidine (DAB) and DAB in combination with 4-chloro-1-naphthol (4CN), which appear as dark blue, brown and black precipitates, respectively. Each substrate has a different affinity for its respective enzyme. Much higher sensitivities can be achieved using NBT/BCIP for AP and 4CN/DAB for HRP as combination substrates.
It is necessary to add Peroxide to the substrate when using HRP conjugates. Traditionally, hydrogen peroxide is added to the buffer prior to use, giving the substrates a shelf-life of only a few hours. Modern formulations offer substrates in stabilized peroxide, which can provide more consistent results in a ready-to-use application.
Band Development and Intensity
The sensitivity of the substrate and the length of incubation determines the intensity of colored precipitate deposited onto the membrane. Color development is monitored visually until it reaches satisfactory strength, following which the reaction may be stopped by washing excess substrate from the membrane or by applying a stop buffer to halt the reaction. The speed of signal development is dependent on the abundance of the reporter enzyme to catalyze the reaction, which is governed by the abundance of the target protein and the primary antibody bound to it.
Less target protein results in fewer antibody complexes binding and consequently less conjugate. Poorly expressed proteins may require prolonged incubations with the substrate of (up to) several hours or even overnight to produce a signal with suitable intensity.
However, this may lead to unacceptable background signal. The developed membrane can be imaged using a CCD camera that enables the bands’ intensity to be analyzed using software such as Image J. Although colorimetric detection cannot provide reliable quantitative data, blots developed under the same conditions/timings may be compared qualitatively.
Inhibition of Enzyme ActivityHRP activity can be inhibited by the presence of sodium azide and contaminants from low-grade glycerol. Therefore, when using this enzyme for visualization, the membrane must be thoroughly washed to remove sodium azide. If glycerol is required, analytic (ACS) grade material should be used to prevent false negatives. Endogenous peroxidases may be found in tissue and can interact with HRP conjugates leading to background signals. Pre-treatment of the sample with hydrogen peroxidase will exhaust endogenous enzyme activity and prevent interference with target protein detection. |
Chemiluminescent Detection
Chemiluminescent detection uses Alkaline Phosphatase (AP) and Horseradish Peroxidase (HRP) reporter enzyme conjugates to enable visualization of the target protein. In chemiluminescent detection, the reporter enzyme catalyzes the development of a luminescent product that emits light instead of a colored precipitate (see Figure 2 below). The signal generated by the target protein(s) is then captured using an X-ray film or a CCD digital imager.
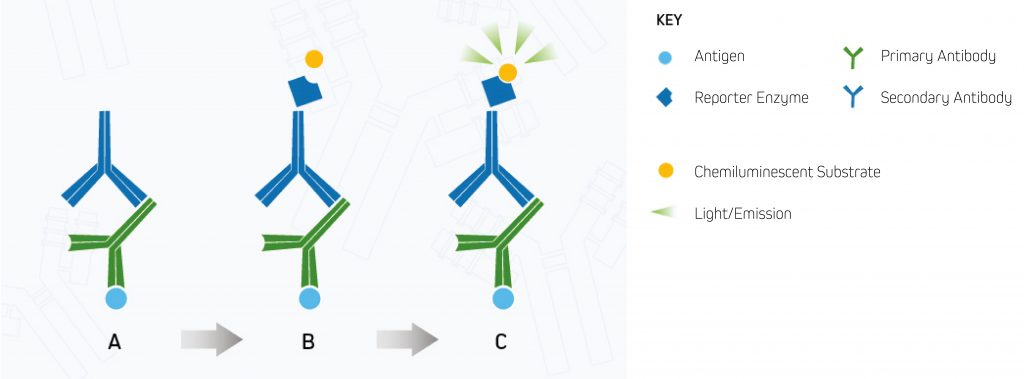
A.Enzyme-conjugated secondary antibody is bound to the primary antibody at the protein of interest.
B.The chemiluminescent substrate is added to the antibody-antigen complex.
C.The reporter enzyme catalyzes the substrate to a light-emitting product, and the signal can be detected by (CCD) digital imager or x-ray film.⁸
Substrates for Chemiluminescent Detection
Compared to colorimetric detection, signal development using chemiluminescent substrates is much quicker, requiring only brief exposures for under a second to capture signal. Luminescence intensity from the bands is captured by either exposure to an X-ray film or using a CCD camera.
Alkaline Phosphatase
Alkaline Phosphatase dephosphorylates its substrate disodium-chloro-5-(4-methoxyspiro{1,2-dioxetane-3,2′-(5′-chloro)tricyclodecan}-4-yl)-1-phenyl phosphate) (CPD-Star®), converting it into the luminescent product, metastable dioxetane phenolate anion. The product emits light at 466 nm and is stable for up to 24 hours.
Horseradish Peroxidase
Horseradish Peroxidase catalyzes its substrate luminol’s oxidation and conversion, in combination with peroxide reduction, into the product 3-aminophthalate, which subsequently emits light at a wavelength of 425 nm.
Enhanced Chemiluminescence (ECL)
The sensitivity of chemiluminescent visualization can be enhanced by choosing high-quality reagents such as Enhanced Chemiluminescence (ECL) substrates. These reagents can increase the sensitivity achieved up to a 1000-fold.
The increased sensitivity offered by ECL substrates enables the visualization of proteins to as low as femtogram levels, allowing the detection of poorly expressed target proteins by X-ray film or CCD camera imaging.
Charged-Coupled Device (CCD) Camera
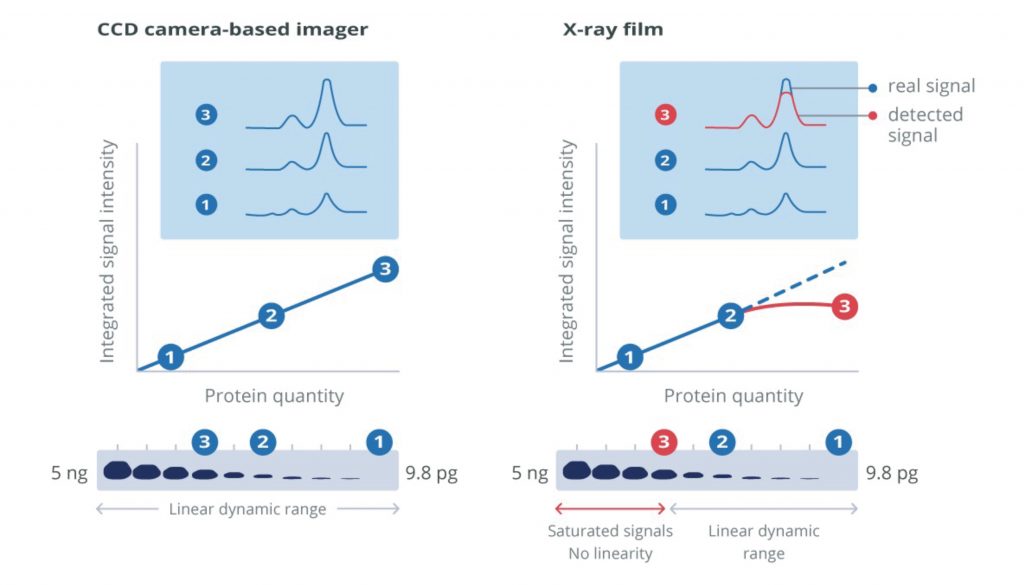
X-ray film or cooled charge-coupled device (CCD) cameras are required to capture signal from blots developed using chemiluminescent or fluorescent report molecules. CCD cameras offer a broad linear dynamic range that can be used for protein quantification and qualitative analysis. Digital imaging with a CCD camera simplifies exposure optimization. Some systems allow manual signal capture and exposure modification which enables the user to tune image capture in real-time. This ability to set parameters means that direct comparisons of conditions can be made, and fine-tuning can optimize the quality of the blots.
Other systems may offer an auto-exposure facility, minimizing the time spent on the optimization process but preventing the user from generating blots that focus on the desired bands. Digital imagers eliminate the use of hazardous developing reagents and costs associated with materials like x-ray film and chemical disposal. The broad dynamic range of CCD cameras and digital output format makes protein quantification possible.
| Type of Detection | Advantages | Disadvantages |
|---|---|---|
| Colorimetric | Inexpensive | Low sensitivity |
| Single output only | ||
| Chemiluminescent | Fast | Single output only |
| Flexible exposure times | Chemical waste produced when using X-ray film | |
| Enhanced chemiluminescence | High sensitivity (1000-fold increase on chemiluminescence) | Semi-qualitative only |
| Multiplex detection with stripping and reprobing | Chemical waste produced when using X-ray film | |
| Fluorescence | Multiplex detection
Qualitative and quantitative |
Specialist equipment for analysis |
Film Capture
X-ray film capture can offer the highest detection sensitivity of all visualization methods. However, the procedure can be wasteful during the optimization process, resulting in large volumes of wasted material from repeated exposures. Because the luminescent product’s emission diminishes over time, reimaged blots can produce weaker intensity bands compared to the initial exposures, making optimization difficult.
Visualization of high- and low-abundance proteins on the same blot can be problematic if the signal intensity from the more abundant protein saturates the film. Initial blots can be performed to assess expression, and samples can be diluted for subsequent blots. This may not be a problem for expression screening, but it can make quantification unreliable and so should be used qualitatively. Substrates that generate stable products with extended signal emission are available and can facilitate repeated exposures.
Detecting Multiple Proteins with Chemiluminescent Detection
Multiple proteins can be detected using ECL but require the membranes to be stripped after visualization of the first protein before reprobing with a second pair of antibodies targeting the next protein of interest. A stripping buffer is used to remove the 1st pair of antibodies and is usually a harsh process that may remove some of the target proteins. This affects the sensitivity of the second probing. Consequently, stripped and reprobed blots are unsuitable for quantitative analysis. A more reliable method of multiplex detection can be achieved using fluorescent detection.
Fluorescent Detection
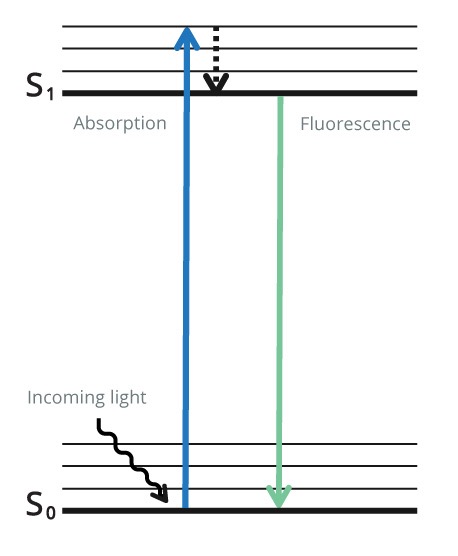
Indirect Fluorescent detection uses a fluorescent probe conjugated to a secondary antibody to visualize the protein of interest. Unlike with colorimetric and chemiluminescence, after the secondary antibody has been added, no further steps are required, and the blot can be immediately analyzed. CCD camera-based imagers or multichannel fluorescent scanners enable qualitative and quantitative data to be obtained. The combination of fluorophores with different excitation and emission spectra can be used for multiplex detection, allowing multiple proteins’ visualization on one blot. Initially, the fluorophore is in its ground state as it does not emit light.
The fluorophore is excited using light at a specific wavelength for the particular fluorophore (excitation wavelength).
The fluorophore absorbs photon(s) and promotes an electron to a higher electronic state (the excitation state). As the electron returns to its ground state, the fluorophore emits energy in the form of a photon of light at lower energy to the excitation wavelength (emission wavelength). The emitted light is detected by a digital imager. This process is shown in Figure 4 below.
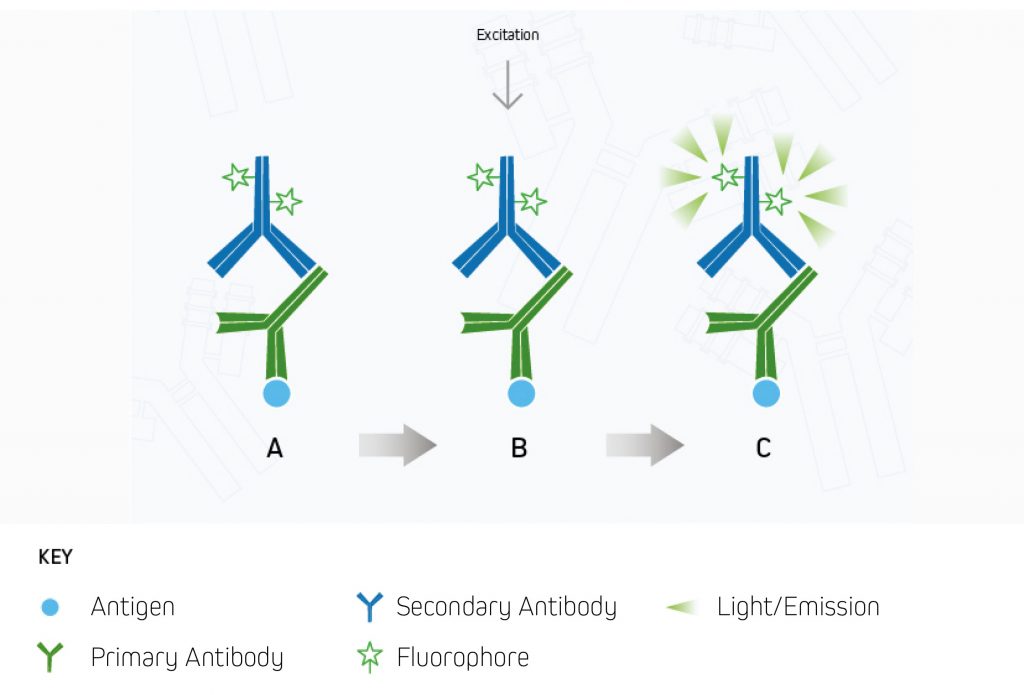
A.The fluorescent dye-conjugated secondary antibody detects the primary antibody for the protein of interest.
B.Light is used to excite the fluorescent conjugate at its excitation wavelength.
C.The excited fluorescent dye emits light at its emission wavelength, which is detected via a digital imager, allowing visualization of the protein of interest.⁹
| Type of Fluorescent Probe | Properties of the Fluorescent Probe |
|---|---|
| Alexa Fluor® Dyes | These dyes have high brightness and photostability. They are water-soluble, remain fluorescent from pH 4–10, and cover the visible to the near infra-red electromagnetic spectrum, making them popular dyes to use. |
| BD Brilliant Violet™ Dyes | BD Brilliant Violet dyes consist of polymer chains and a bright fluorescence signal, with high resolution and sensitivity. |
| Cyanine™ Dyes | These have a high photostability and wide pH tolerance (pH 3–10) |
Detecting Multiple Target Proteins on the Same Blot – Multiplex Detection
Each fluorophore has its own spectral characteristics, enabling the simultaneous detection of multiple target proteins.
Multiplex detection can be performed without the need for stripping and reblotting, preventing damage to the target protein.
Each target protein is identified by a primary antibody of a unique host species or isotype, which is then detected using a fluorophore-conjugated secondary antibody with its own emission wavelength enabling each protein to be imaged and distinguished simultaneously.
Dye Selection
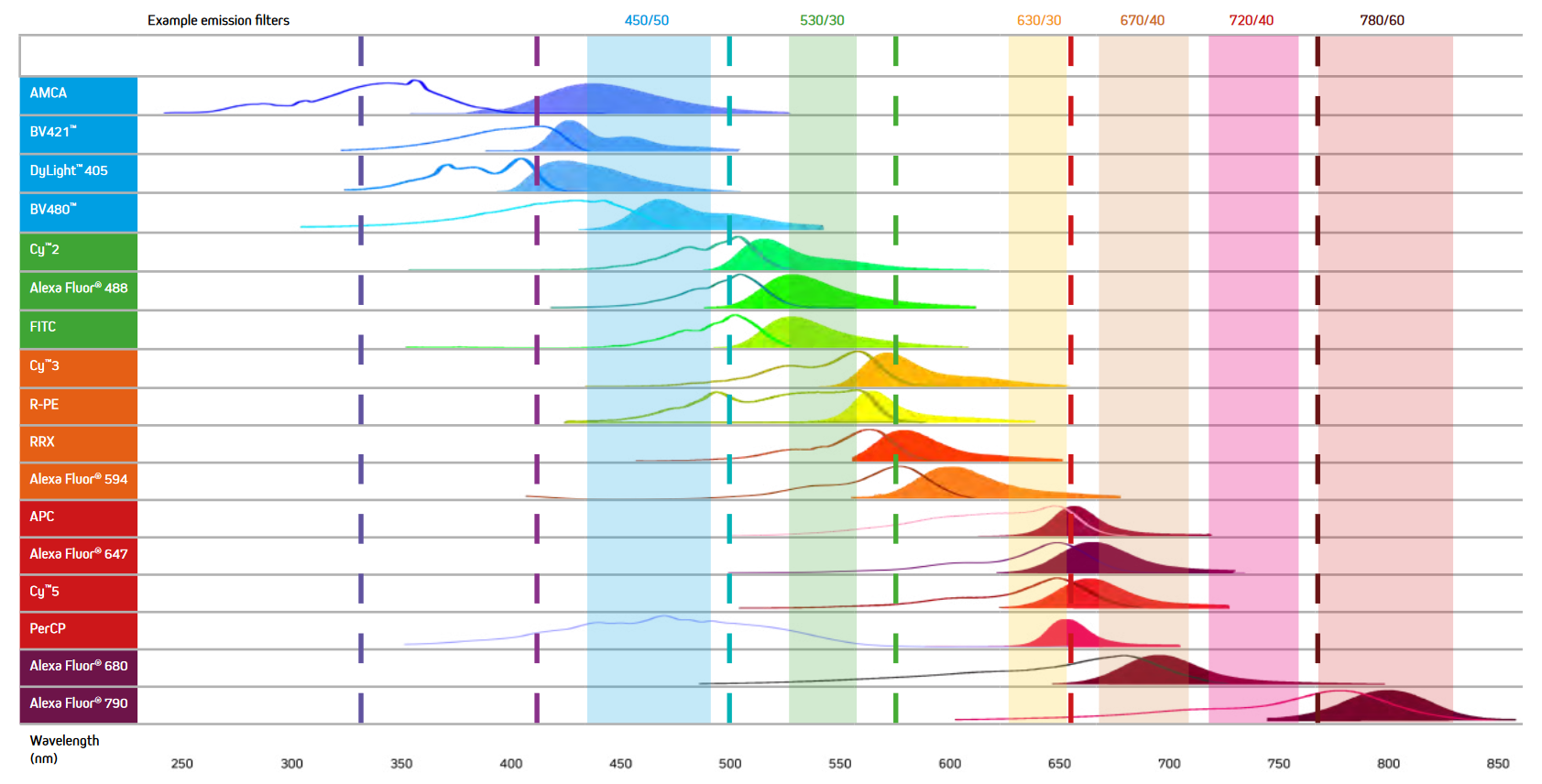
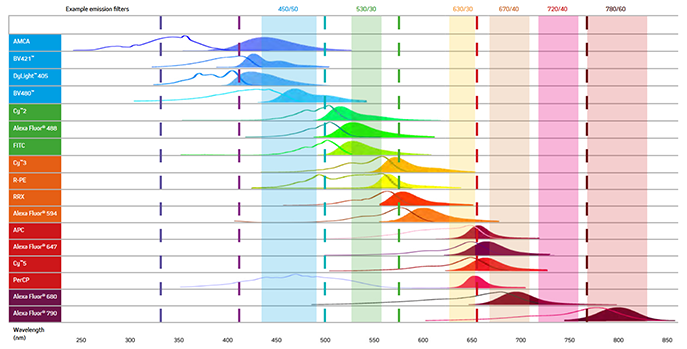
When selecting the fluorescent probes for multiplex imaging, it is essential to choose dyes with minimal spectral overlap (See Figure 7). For example, while the excitation and emission maxima of AMCA and BV421 are different, they still suffer from spectral overlap, and as a result, the signal from two proteins labeled with them could not be distinguished, making the use of these dyes together unadvisable. Figure 7 below shows the excitation and emission spectra of a range of fluorescent probes offered by Jackson ImmunoResearch.
Lenses and Data Collection
CCD digital imagers use a silicon chip divided into thousands of tiny light-sensitive squares, commonly referred to as pixels. Photons of light travel through the lens of the camera to reach the pixels. As a photon strikes the chip, an electron is released and then stored. The number of electrons stored in each pixel is directly proportional to the signal intensity of the image that is generated.
The sensitivity of CCD imagers is limited by the number of pixels the chip contains. Generally, a larger pixel number will enable higher sensitivity, although this may be affected by how the chip processes the signal it receives. The lens can either be fixed or have a zoom capacity; however, the zoom capacity tends to decrease the sensitivity, and so fixed lenses are recommended. The lens aperture dictates the amount of light that can pass through to the detector, and so larger apertures should be used when imaging weak signals.
Multichannel Imagers
CCD digital imagers are designed for chemiluminescence and fluorescence detection. A light source is used to illuminate the membrane or excite fluorophores at specific wavelengths. For fluorescent applications, a number of excitation sources can be used, including white light gas discharge tubes, xenon arc lamps and high-intensity light-emitting diodes.
Higher sensitivity can be achieved using scanning imaging systems. They exploit a laser’s narrow beam of highly monochromatic light. In addition, by using beam splitters and filters to focus specific wavelengths of light on a sample and/or to detect particular wavelengths from a sample, multichannel detection can be performed. This allows for the simultaneous detection of multiple fluorophores using a single, tunable light source.
Autofluorescence
Fluorescent probes used in early western blots initially gave a poor performance as only fluorophores with emission in the visible region were used.
Fluorescent probes which are excited in the visible region can cause auto-fluorescence – fluorescence of biological molecules present in the cell or tissue sample. The unwanted fluorescence prevents analysis of results.
PVDF membranes can autofluoresce, creating a signal which can prevent the identification of the target signal. Low-fluorescence PVDF membranes should be used for fluorescent western blotting.
Other sources of fluorescent interference include labeling the membrane with a ballpoint pen or handling the blot with powdered gloves, which should be avoided.
Far-Red and Infrared Dyes
Fluorophores that emit in the far red or infra-red region, such as Alexa Fluor® 680 and 790, enable researchers to visualize proteins at wavelengths not commonly associated with autofluorescence as the emission wavelength is far from the visible spectrum.
Far-red emitters exhibit low fluorescence quenching and have high extinction coefficients. These characteristics enable fluorescent western blotting to achieve similar sensitivity to ECL detection, with the advantage of a wide linear dynamic range for protein quantification.
Stable fluorescent probes may also enable blots to be stored and re-imaged later if they are protected from light.
References
- GE Healthcare. (2011). Western Blotting Principles and Methods. https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma-Aldrich/General_Information/1/ge-western-blotting.pdf
- Bass J.J. et al. (2017). An Overview of Technical Considerations for Western Blotting Applications to Physiological Research. Scandinavian Journal of Medicine and Science in Sports. DOI: 10.1111/sms.12702
- Jackson ImmunoResearch Laboratories Inc. (2017). Western Blotting Troubleshooting Guide! https://www.jacksonimmuno.com/secondary-antibody-resource/technical-tips/western-blot-trouble-shooting/
- Jackson ImmunoResearch Laboratories Inc. (2020). Specializing in Secondary Antibodies and Conjugates. https://www.jacksonimmuno.com/files/Jackson-ImmunoResearch-Secondary-Antibody-Catalog.pdf
- Lewis M. (2016). Chromogenic Detection for Western Blot, IHC, and ELISA. Jackson ImmunoResearch Laboratories Inc. https://www.jacksonimmuno.com/secondary-antibody-resource/immuno-techniques/chromogenic-detection-for-western-blot-ihc-and-elisa/
- Lewis M. (2016). Colorimetric Western Blotting. Jackson ImmunoResearch Laboratories Inc. https://www.jacksonimmuno.com/secondary-antibody-resource/immuno-techniques/colorimetric-western-blotting/
- Lewis M. (2016). Chemiluminescent Western Blotting. Jackson ImmunoResearch Laboratories Inc. https://www.jacksonimmuno.com/secondary-antibody-resource/immuno-techniques/chemiluminescent-western-blotting/
- Lewis M. (2016). Fluorescent Western Blotting. Jackson ImmunoResearch Laboratories Inc. https://www.jacksonimmuno.com/secondary-antibody-resource/immuno-techniques/fluorescent-western-blotting/
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



