3 methods of detection are available for Western blotting: colorimetric, chemiluminescent and fluorescent. Each detection method can offer advantages, in terms of sensitivity, quantification, cost-per-assay or multiplex detection.
Western blotting is an analytical technique used to identify specific proteins from biological samples or solutions. Linearized proteins are separated by gel electrophoresis to resolve them by size and then transferred onto a membrane such as nitrocellulose or polyvinylidene difluoride (PVDF) which immobilizes the protein. The membrane is probed with a primary antibody directed against the specific protein of interest. A secondary antibody conjugated to a reporter molecule is then used to identify the primary antibody and produce the desired signal.
Colorimetric detection
Alkaline Phosphatase (AP) and Horseradish Peroxidase (HRP) conjugates can be used for colorimetric detection (Figure 1(A)). The conjugated reporter enzyme catalyzes the conversion of the chromogenic substrate to a colored precipitate, visualized directly on the blotting membrane. Colorimetric detection can offer quick and easily obtained results without the need for expensive detectors or extensive optimization.
Chemiluminescent detection
Enzyme-linked conjugates can also be used for chemiluminescent signal detection (Figure 1(B)). HRP conjugates produce signal by oxidizing a chemiluminescent substrate (luminol) to a form which emits light.
AP conjugates produce signal when the enzyme dephosphorylates a specific substrate (e.g. 1,2-dioxitane) to a light emitting product.
The signal can then be captured by exposing photographic film to the membrane or using a cooled charge-coupled device (CCD) camera. Chemiluminescent detection offers excellent sensitivity, however quantification and probing for multiple targets can be limited, and development may require refinement to optimize signal capture.
Fluorescent detection
Fluorescent signal detection uses a fluorescent dye conjugate to visualize antigen on the membrane (Figure 1 (C)). Light at a wavelength specific to the dye’s spectral characteristic is used to excite the fluorescent dye. The absorbed light excites the dye’s electrons to a higher electronic state, and as they return to their ground state they emit photons at the emission wavelength characteristic of the fluorophore. The light is detected by a digital imager fitted with appropriate filters. Fluorescent Western blotting allows for quantitative analysis and multiplex probing without the need for stripping and reblotting.
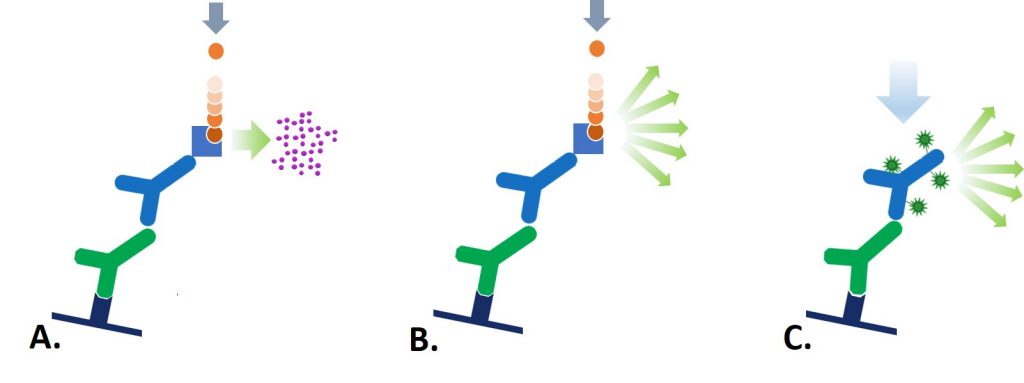
Figure 1: The 3 detection methods for Western blot: (A) Colorimetric, (B) Chemiluminescent, and (C) Fluorescent; A. The reporter enzyme conjugate catalyzes the conversion of a chromogenic substrate to a colored insoluble precipitate, visible by eye on the blotting membrane. B. The reporter enzyme conjugate catalyzes a reaction which converts the chemiluminescent substrate to a light emitting form,and the emitted light is detected by X-ray film or CCD camera. C. The reporter fluorescent dye is excited by its characteristic wavelength light, and resulting emitted light is captured by a digital imager.
A brief overview of the detection methods are detailed in the table below.
| Colorimetric | Chemiluminescent | Fluorescent | |
| Signal-stability | Stain is very stable, will not fade under examination and can be stored with minimal loss of color for years. | Signal may last for minutes to hours – but may be restored by additional incubation with chemiluminescent substrate. | Fluorescent dyes have limited longevity under examination and after storage. |
| Sensitivity | Low | High | Medium-high, depends on detection instrument |
| Quantitative | No | Semi-quantitative | Yes |
| Multiplexing | Single probing – multiple proteins on one blot require stripping and reprobing | Single probing – multiple proteins on one blot require stripping and reprobing | Simultaneous detection of multiple antigens is possible without striping and re-probing. Overlapping migrations can be determined in the individual color channels and presented digitally, either as a merge or separately. |
| Dynamic range | 10-50 fold | >4000 fold | |
| Equiptment | No special equipment | Film developer | Digital imager |
Table 1: Features of detection methods – advantages and disadvantages
For more detailed information about specific detection techniques please follow the links below
References:
Alberts B et al (1994) Molecular biology of the Cell. 3rd Ed. Garland press. London
Roitt et al (2005) Immunology. 6th Ed. Mosby. Spain


