Here, we discuss the differences between the different antibody formats available, including H+L, F(ab′)2, Fab, and VHH fragment antibodies. We will address how they can offer solutions to your experimental setup, help you improve specificity and resolution, and circumvent limitations.
Antibody Formats – not just the heavy and light chain
Jackson ImmunoResearch offers Affinity-Purified secondary antibodies in four different formats. Select from Whole IgG, F(ab’)2 fragment, Fab fragment, and VHH fragment antibodies. The optimal format for your experiment depends largely on the intended application and expectations. Whole molecule antibodies, comprised of both heavy and light chains, making up the Fab and Fc domains, are suitable for most immunodetection procedures. However, the specialist formats of F(ab’)2, Fab, and VHH antibodies can offer advantages to users with specific assay requirements.
Whole-molecule antibodies
Canonical or conventionally shaped immunoglobulins are antibodies with heavy and light chains that make up the easily recognizable Y shape. The Y shape is a monomeric subunit of the immunoglobulin, even if both chain species aren’t present (see camelid antibodies below). The antibody may exist as a Y monomer in the immunoglobulin classes D, E, and G. Whereas Ig class A exists as both a monomer and a dimer, and IgM as a pentamer or hexamer.
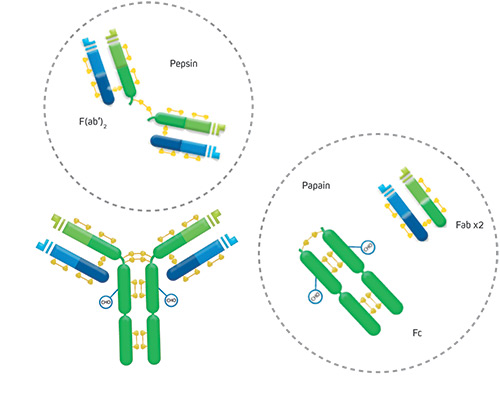
The arms of the Y consist of two antigen-binding regions called a Fab (Fragment antigen binding) fragment. The Fab arms are joined together by disulfide bonds (Figure 1). With the exception of many engineered antibodies (learn more), the two arms are typically specific (bind) to the same epitope, making the antibody divalent.
The trunk of the antibody is made up of the Fc (fragment crystallizable) region or domain. This Fc domain confers the antibody’s functionality in its biological setting. Specific receptors bind to it, activating downstream pathways.
The whole Ig molecule is a commonly used antibody format suitable for most immunoassays and immunodetection applications. IgG is roughly 160 kDa, and the other classes vary slightly in their monomeric subunit.
F(ab’)2 Fragment antibodies
Another format that antibodies can be found in is as an F(ab’)2 fragment antibody. These comprise the two Fab arms linked by part of the antibody hinge. The F(ab’)2 is generated by limited proteolysis using pepsin digestion of whole IgG antibodies. The digestion removes most of the Fc region whilst leaving some of the hinge region intact. Consequently, the two Fab arms/fragments are linked to create the F(ab’)2 fragments, making them divalent. Their average molecular weight is about 110 kDa.
F(ab’)2 antibodies are generally used for specific applications due to the absence of the Fc domain. One application is when it is necessary to avoid the secondary antibody binding to live cells via their Fc receptors. Consequently, they are popular in flow cytometry or FACS (fluorescence-activated cell sorting) experiments where avoiding internalization or stimulation of various cell types is desirable. F(ab’)2 antibodies can offer the same specificity, cross adsorption, and conjugate options as whole molecule antibodies, but because the F(ab’)2 format is popular for flow cytometry applications, they are often available conjugated to fluorescent proteins such as PerCP, APC, and R-PE. These large fluorescent proteins are ideal for surface staining and can provide excellent signal. F(ab’)2 antibodies can also be useful to avoid binding Protein A or G.
Caution: Primary antibodies can still bind to Fc receptors expressed on the sample cells’ surface if the primary antibodies are whole IgG format antibodies. This binding can generate background signal/ non-specific signal regardless of the format of the secondary antibody.
To avoid Fc receptor binding, generating background blocking is necessary. Simply incubate cells in buffer solution containing 5% normal serum from the host species of the labeled secondary antibody.
To prevent endocytosis (called capping) and the regeneration of Fc receptors on living cells, incubate cells at 4°C in buffer containing 5% normal serum and sodium azide added to inhibit metabolism.
Fab Fragment antibodies
As we learned above, a Fab fragment antibody is a single binding arm of the whole immunoglobulin molecule. Fab fragments are also generated with limited proteolysis, but the enzyme used is papain. Digestion of whole IgG antibodies with papain removes the entire Fc domain, including the hinge region (Figure 1). Consequently, Fab fragment antibodies are monovalent, with a molecular weight of about 50 kDa.
Fab fragments can be very useful tools in addition to standard primary and secondary antibodies. One use is to block endogenous immunoglobulins on cells, tissues, or other surfaces in which the primary antibody is the same species as the tissue. Blocking with Fab fragments helps prevent non-specific signals and promotes the specific detection of the primary antibody by the secondary antibody. In multiple labeling experiments, Fab fragments can facilitate the success of experiments using two primary antibodies generated in the same host species.
FabuLight™
FabuLight™ antibodies are Fab fragment antibodies specific to the Fc region of IgG or IgM primary antibodies available conjugated to a range of reporter molecules. FabuLight antibodies enable primary antibodies to be labeled before incubation with cells or tissue. This avoids the active site of the primary antibody being compromised and can make the protocol more efficient by removing an incubation and wash step. FabuLights are ideal for flow cytometry experiments where it is desirable to avoid Fc receptor binding. The Fc domain is absent from the FabuLight, enabling labeling of cell surface immunoglobulins without risk of stimulation/activation of cells.
Camelid antibodies and VHH antibodies (NANOBODIES®)
Camelid species such as Alpacas and Llamas produce a unique class of antibodies composed only of heavy chains (Figure 2b). Light chains are absent from IgG2 and 3 antibodies of camelid species. The immunological function of these unconventional-format antibodies is still being investigated. A consequence of the lack of light chains is that the antigen-binding fragments (Fab), also termed Variable heavy-chain-only fragment antibodies (VHH Fragments), or NANOBODIES® (Figure 2a), have some tractable characteristics for life science applications.
One of the advantages of the absence of the light chain is that VHH fragments are very small. Another happy accident is that they are extremely stable as both a cleaved native protein and as a recombinant construct. So easily produced recombinantly, the VHH format antibody is fast becoming a popular framework for therapeutic mAbs and ADC (antibody-drug conjugates). As a primary or secondary antibody, the VHH antibodies are an exciting, novel antibody format with outstanding specificity and penetration, and offer a fantastic solution for high-quality and high-resolution imaging.

AffiniPure-VHH® Secondary Antibodies
Jackson ImmunoResearch AffiniPure-VHH® Secondary Antibodies are polyclonal VHH Fragment antibodies (NANOBODIES®) produced in Alpacas. They are available with specificity to Human, Rabbit or Mouse, and are cross-adsorbed against other commonly used species. They are 10x smaller than conventional whole IgG antibodies, and are available conjugated to reporter enzymes and a range of fluorescent dyes, including Alexa Fluor®. They provide scope for high-resolution Immunohistochemistry and Immunofluorescence by reducing linkage error between the target antigen and signal-producing dye.
References
- Shimomura O, Johnson FH, Saiga Y. Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea. J Cell Comp Physiol. 1962;59:223-239. doi:10.1002/jcp.1030590302 https://pubmed.ncbi.nlm.nih.gov/13911999/
- Heim R, Cubitt AB, Tsien RY. Improved green fluorescence. Nature. 1995;373(6516):663-664. doi:10.1038/373663b0 https://pubmed.ncbi.nlm.nih.gov/7854443/
- Heim R, Prasher DC, Tsien RY. Wavelength mutations and posttranslational autoxidation of green fluorescent protein. Proc Natl Acad Sci USA. 1994;91(26):12501-12504. doi:10.1073/pnas.91.26.12501 https://pubmed.ncbi.nlm.nih.gov/7809066/
- Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP). Gene. 1996;173(1 Spec No):33-38. doi:10.1016/0378-1119(95)00685-0 https://pubmed.ncbi.nlm.nih.gov/8707053/
- Romei MG, Boxer SG. Split Green Fluorescent Proteins: Scope, Limitations, and Outlook. Annu Rev Biophys. 2019;48:19-44. doi:10.1146/annurev-biophys-051013-022846 https://pubmed.ncbi.nlm.nih.gov/30786230/
- Kodama Y, Hu CD. Bimolecular fluorescence complementation (BiFC): a 5-year update and future perspectives. Biotechniques. 2012;53(5):285-298. doi:10.2144/000113943 https://pubmed.ncbi.nlm.nih.gov/23148879/
- Ghosh I, Hamilton AD, Regan L. Antiparallel Leucine Zipper-Directed Protein Reassembly: Application to the Green Fluorescent Protein. J Am Chem Soc. 2000;122:5658-5659. DOI:10.1021/JA994421W https://www.semanticscholar.org/paper/Antiparallel-Leucine-Zipper-Directed-Protein-to-the-Ghosh-Hamilton/84e65b5eb57f93deadee8983bd67315c1a980561
- Cabantous S, Nguyen HB, Pedelacq JD, et al. A new protein-protein interaction sensor based on tripartite split-GFP association. Sci Rep. 2013;3:2854. doi:10.1038/srep02854 https://pubmed.ncbi.nlm.nih.gov/24092409/
- Pham, TD, Poletti, C, Tientcheu, TMN et al. FAST, a method based on split-GFP for the detection in solution of proteins synthesized in cell-free expression systems. Sci Rep. 2024;14:8042. https://doi.org/10.1038/s41598-024-58588-5