
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
They are available conjugated with 9 different fluorophores and biotin, and enable labeling of primary antibodies prior to incubation with cells or tissue. This provides a time-saving alternative to sequential incubation flow cytometry and immunohistochemistry procedures, without compromising the active site of the primary antibody.
FabuLights can be used to label Fc domains of fusion proteins, or cell surface immunoglobulins, without cross-linking or activating cells.
FabuLights are not cross-adsorbed against other species, so blocking steps may be required to avoid labeling endogenous immunoglobulins. For advice on developing protocols, refer to the example protocol overleaf.
Incubation with FabuLight-labeled primary antibodies requires fewer washes than sequential incubation with primary antibodies and labeled secondary antibodies, thereby reducing damage to cells in flow cytometry protocols. Incubation steps in protocols requiring multiple primary antibodies from the same host animal are also reduced.
FabuLight binds to the Fc portion of the primary antibody (either anti-IgG, Fcγ specific or anti-IgM, μ chain specific), leaving the antigen-binding region active.
The resulting complex of primary antibody with FabuLight does not precipitate or aggregate, because the dye-conjugated Fab anti-Fc secondary antibody fragments are monovalent. FabuLight-primary antibody complexes offer good tissue penetration and low non-specific binding.
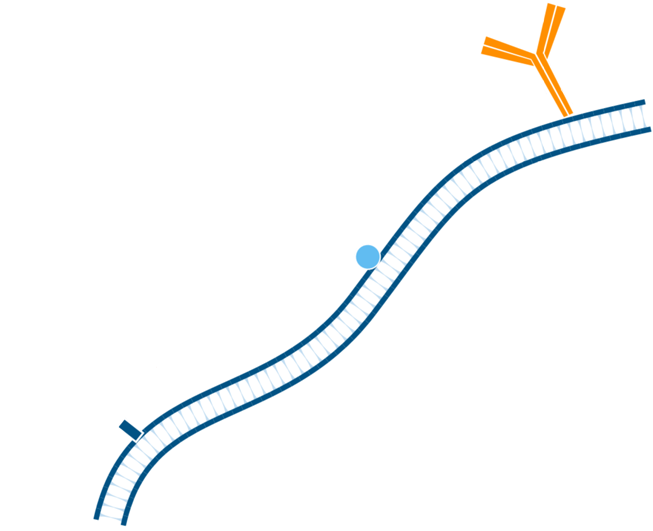
Step 1. Include a blocking step if the tissue of interest displays endogenous immunoglobulin.
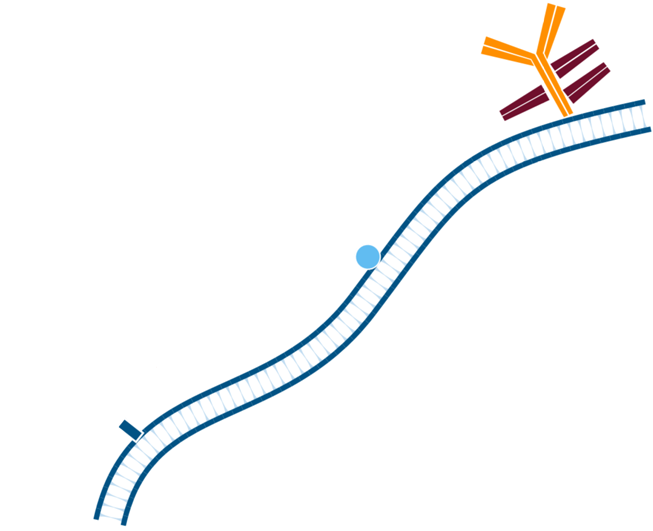
Step 2. In this instance, mouse tissue will be labeled using primary antibodies raised in mouse. Pre-incubate tissue/cells with unconjugated Fab anti-Fc antibody to block endogenous immunoglobulin, in this example the Fab used is Goat Anti-Mouse IgG1, Fcγ fragment specific. Wash.
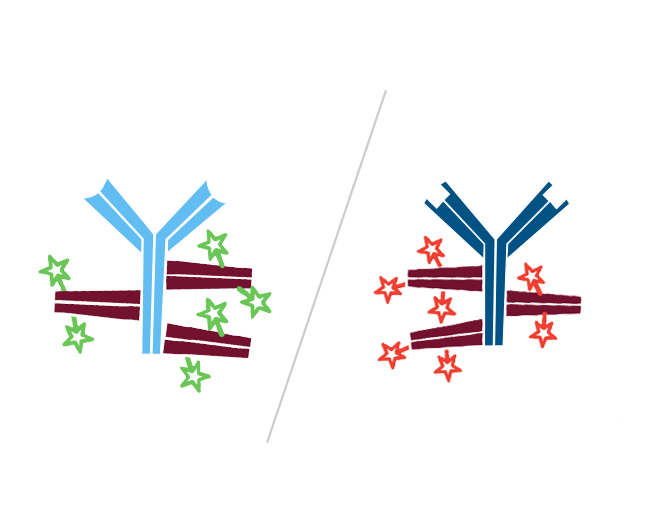
Step 3. Create FabuLight-primary antibody complexes. In this example, Mouse Anti-Antigen X is complexed with an Alexa Fluor® 488-conjugated Fabulight (Goat Anti-Mouse IgG1, Fcγ fragment specific (115-547-185)); and Mouse Anti-Antigen Y is complexed with Rhodamine Red-X-conjugated FabuLight (Goat Anti-Mouse, IgG1 Fcγ fragment specific (115-297-185)).
Step 4. This creates two antibody:FabuLight complexes specific for antigen X and Y.
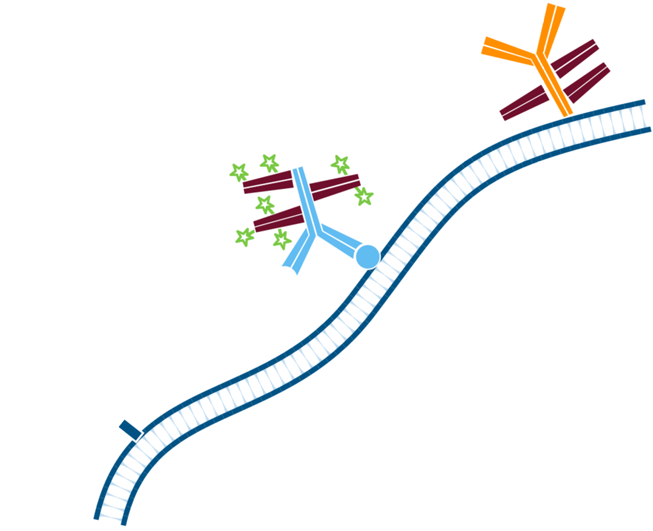
Step 5. The least abundant target is incubated first with the Mouse Anti-Antigen X complexed with Alexa Fluor® 488-conjugated FabuLight (Goat AntiMouse IgG1, Fcγ fragment specific). Wash.
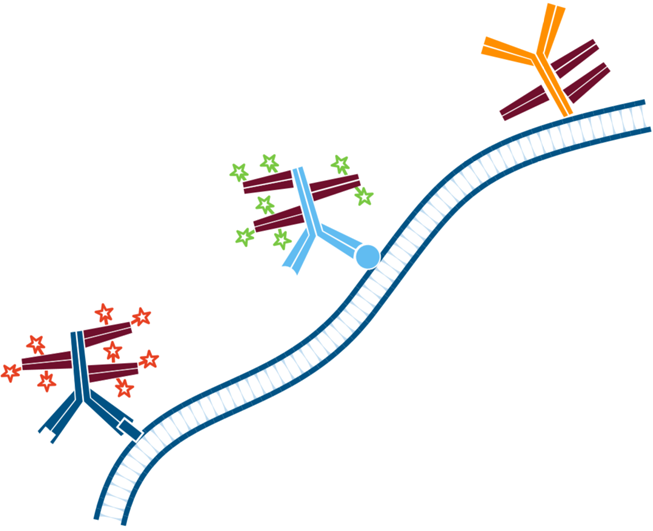
Step 6. The sample is then incubated with the Mouse Anti-Antigen Y (more abundant target) complexed with Rhodamine Red-X conjugated FabuLight (Goat Anti Mouse IgG1, Fcγ fragment specific). Wash.

Figures 1-5 show how optimized conditions can improve multiple labeling protocols. Titrate Fab-labeled complexes vs. their target antigens to minimize interactions from unbound complexes. Figures 1-2 demonstrate that positive signal can be maintained under diluted conditions. Sequential incubations, starting with the less abundant antigenic target, provide the best discrimination between the antigens (cell types) as shown in Figures 3-4. The Fab-labeled complexes can also be incubated simultaneously if they have been properly titrated (Figure 5).
Complexes were formed at a 3:1 molar ratio (equal weight ratios) of FabuLight:primary antibody. Alexa Fluor® 488 Fab Goat Anti-Mouse IgG1, Fcγ fragment specific (115-547-185) was complexed with Mouse Anti-Human CD3 (BD 555330)(AF488/CD3); and Alexa Fluor® 647- Fab Goat Anti-Mouse IgG1, Fcγ fragment specific (115-607-185) was complexed with Mouse Anti-Human CD19 (BD 555410)(AF647/ CD19).
Blood was collected from normal human donors and treated with lysis buffer. Cells were centrifuged, washed, and resuspended in isotonic PBS + 0.5% BSA. They were then incubated with complexed antibodies for 30 minutes, washed, and analyzed on a BD FACSCalibur flow cytometer gated on lymphocytes.
Figures 1 and 2 illustrate insignificant signal loss with diluted reagents. For multiple labeling protocols, excess reagent will result in mislabeling of antigens as illustrated in Figures 3-5. Use the lowest concentration possible to optimize results.
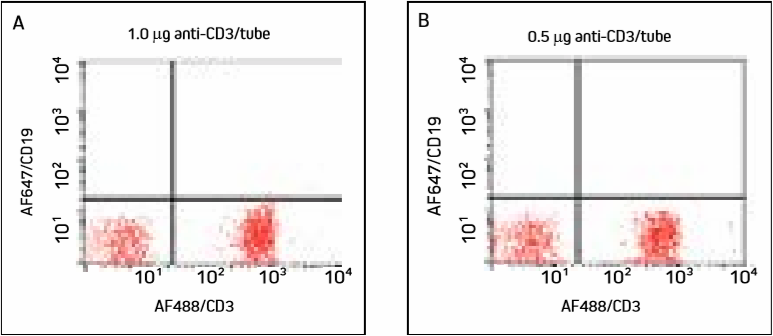
Figure 1. Cells were incubated with two different concentrations of AF488/CD3 and washed. Panels A and B show a large population of T cells in the lower right quadrant, with similar detection at both concentrations.
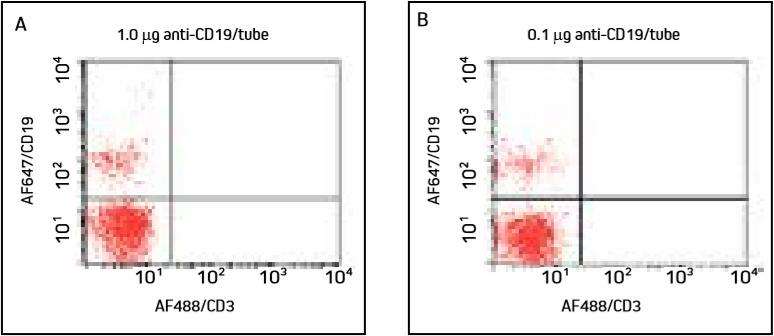
Figure 2. Cells were incubated with two different concentrations of AF647/CD19 and washed. Panels A and B show a smaller population of B cells in the upper left quadrant, with similar detection at both concentrations.
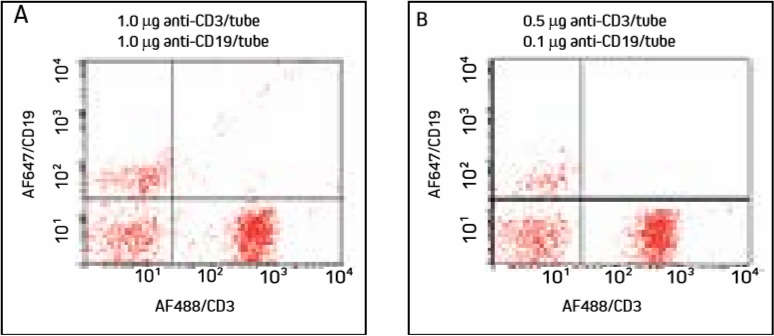
Figure 3. Cells were incubated with AF647/CD19 (primary to the less abundant target used first), washed, then incubated with AF488/CD3 and washed. Note specific labeling of T cells in the lower right quadrant and B cells in the upper left quadrant.
Targeting the less abundant antigenic site first separates the cell populations well. Diluted antibodies (Panel B) produce fewer double labeled cells, with insignificant signal differences.
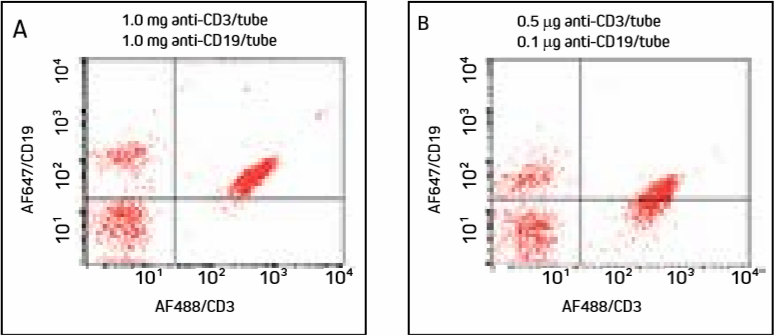
Figure 4. Cells were incubated with AF488/CD3 (primary to more abundant target used first), washed, then incubated with AF647/CD19 and washed.
Note increased double labeling of cells compared with Figure 3.
Panel A shows excess AF647-FabuLight has bound to anti-CD3 on the T cells, shifting the population into the upper right quadrant. Panel B shows that under diluted conditions, T cells have shifted down compared with Panel A. Additonal titration of anti-CD19 may further improve this result, though labeling the less abundant target antigen first (Figure 3) avoids the problem.
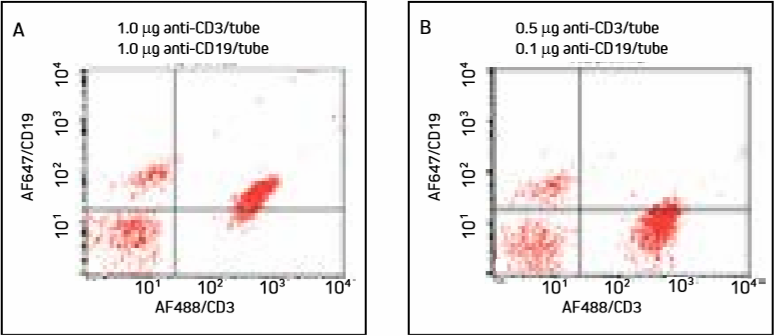
Figure 5. Cells were incubated with a cocktail of AF488/CD3 and AF647/CD19 and washed.
Mislabeling of cell populations is dramatically reduced when antibodies are more dilute.
In Panel A, excess AF647-FabuLight has bound to anti-CD3 on T cells, shifting the T cell population into the upper right quadrant. Panel B shows that diluted antibodies bind more specifically, and T cells remain in the lower right quadrant with insignificant loss of signal.
Follow the correct steps to ensure the best results with FabuLights.
Background staining may be observed if a labeled secondary antibody is not adsorbed to minimize recognition of endogenous tissue Ig.
View Blocking Protocol
Monovalent Fab fragments of affinity-purified secondary antibodies are offered to block the surface of immunoglobulins
Read More