
Allergy affects many people and has multiple causes including pet dander, pollen, and chemical allergens. It may manifest as skin irritations or rhinitis, through to the more serious such as allergic asthma and life-threatening anaphylaxis. Allergy is commonly diagnosed using immunoassays which can identify the causative agent and the disease’s severity. In this article, we introduce allergy and present two immunotechniques commonly used to characterize and diagnose allergies.

Allergy affects the lives of many people globally and is an increasing problem for people living in the northern hemisphere (Zhang and Steiner 2022; Anderegg, W.R. et al., 2021). The incidence of companion animal, food, and environmental allergies appears to be rising and affects a large proportion of the population (Pawankar et al., 2011’ Loh et al., 2018). Beyond allergy’s considerable impact on health and well-being, it has a considerable financial cost. Allergic reactions can range in severity. They can manifest as local symptoms, “atopy,” which causes minor irritation and inconvenience, such as allergic rhinitis or “hayfever.” On the other end of the spectrum, allergic reactions may have more severe systemic manifestations, such as drug or insect venom allergy leading to anaphylactic shock. Not all allergies lead to such severe outcomes. The severity of the reaction is controlled by several factors, including dose, route of exposure, allergen, age, comorbidities, and the immune competence of the individual.
IgE is produced in response to exposure to an allergen and some parasitic infections. IgE is also produced as part of the inflammatory sequelae in some autoimmune disorders. In the case of allergy, the IgE acts functionally to trigger the release of histamine and other agents from mast/specialized cells, initiating what is recognized as the allergic response. Consequently, detection and quantification of specific and total IgE from patient serum is a common clinical procedure in allergy diagnosis. Detection of specific IgE may be used to identify the allergen responsible for sensitivity, such as nut or pollen. Levels of specific IgE from patients can correspond to the severity of the allergic reaction. Therefore, these levels have been used to develop systems to grade the allergy. Several grading systems have evolved to help clinicians standardize observations when clinical features differ between patients (Sanchez-Borges et al., 2019). These grades are standardized and form the foundation of current commercial testing systems.
Here we demonstrate the sensitivity and specificity of JIR Anti-Human IgE to detect specific IgE from human serum in common immunoassays used in the characterization of allergy.
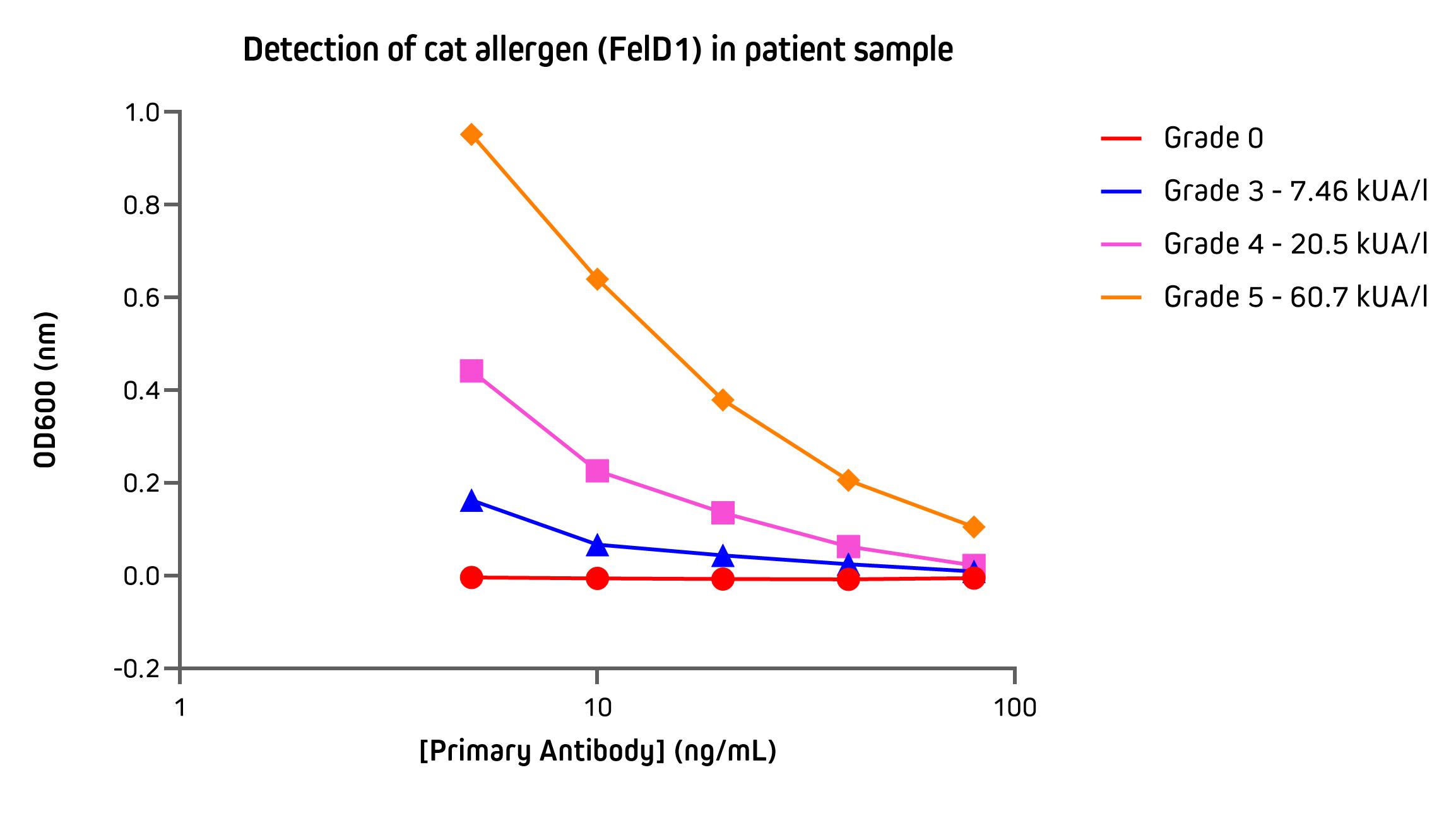
Detection of IgE specific to cat allergen by ELISA
ELISA (Enzyme-linked immunosorbent assay) is a commonly used immunoassay for the detection and quantification of immunoglobulins from biological samples. They may be employed in allergy testing to identify IgE class antibodies specific to allergens, either as part of a screen to identify the sensitizing agent or in efforts to characterize the severity of the allergy.
The assay below uses an ELISA to illustrate the utility of anti-IgE in the detection of IgE specific to cat dander allergen (FelD1) in sera from patients. Patient sera were graded between 0 – 5 by using the Phadia ImmunoCap assay system. These data demonstrate the sensitivity and quantitative response of Alkaline Phosphatase Anti-Human IgE used as the detection reagent.
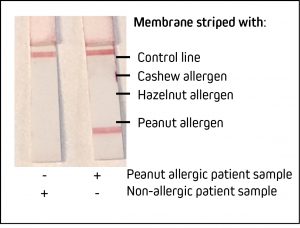
Detecting nut allergy: Detection of IgE specific to Peanut by LFIA
Lateral flow immunoassays (LFIA) are an effective testing method to detect the presence of antigens or antibodies at the point of care. As seen during the recent Covid-19 pandemic, they offer rapid and qualitative “yes or no” results. LFIA or LFTs (Lateral flow tests) are an excellent format to confirm food allergy in a clinical setting. Food allergies can range in severity and can impact the patient’s quality of life (Cox et al., 2010). Accurate and rapid testing can ensure swift diagnosis and access to suitable treatment or prophylaxis. It should be noted that assays should be validated by the user. Here we demonstrate the utility of JIR Anti-Human IgE in a contrived LFIA against the common and potentially serious allergen – peanut.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications, including ELISA and LFIA. Our portfolio includes anti-IgE antibodies conjugated to HRP, Biotin, Alkaline Phosphatase, and R-PE. We’ve also recently expanded our range of gold-conjugated secondary antibodies with the addition of 40 nm colloidal gold conjugates, which are among the most frequently used detection moieties for LFIA.
References:
- Anderegg, W.R. et al., 2021. Anthropogenic climate change is worsening North American pollen seasons. Proceedings of the National Academy of Sciences, 118(7).
- Cox, L., Larenas-Linnemann, D., Lockey, R. and Passalacqua, G., 2010. Speaking the same language: The World Allergy Organization Subcutaneous Immunotherapy Systemic Reaction Grading System. Journal of Allergy and Clinical Immunology, 125(3), pp.569-574.e7.
- Pawankar, R., Canonica, G., Holgate, S., and Lockey, R., 2011. World Allergy Organization (WAO) white book on allergy. United Kingdom: WAO.
- Loh W, Tang MLK. The Epidemiology of Food Allergy in the Global Context. Int J Environ Res Public Health. 2018 Sep 18;15(9):2043. doi: 10.3390/ijerph15092043. PMID: 30231558; PMCID: PMC6163515.
- Sánchez-Borges, M., Ansotegui, I. & Cox, L. World Allergy Organization Grading System for Systemic Allergic Reactions: it Is Time to Speak the Same Language When it Comes to Allergic Reactions. Curr Treat Options Allergy 6, 388–395 (2019). https://doi.org/10.1007/s40521-019-00229-8
- Zhang, Y., Steiner, A.L. Projected climate-driven changes in pollen emission season length and magnitude over the continental United States. Nat Commun 13, 1234 (2022). https://doi.org/10.1038/s41467-022-28764-0
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



