
Flow cytometry is a technique used to analyze individual cells in suspension. It uses a stream of fluid to direct the cells in single file past an interrogation point, where light scatter and fluorescence intensity are measured. This technique enables the characterization of distinct sub-populations of cells to be identified by their expression of specific surface markers using immunostaining with labeled antibodies. Multiple parameters can be analyzed using fluorescently-labeled antibodies combined in a panel to detect multiple markers simultaneously. Here we demonstrate the utility of VHH fragment antibodies for flow cytometry using indirect sequential staining to achieve optimum signal.

Benefits of indirect flow cytometry
Indirect flow cytometry allows researchers to utilize the signal-enhancing properties of conjugated secondary antibodies. Secondary detection of the antibody targeting the protein of interest brings multiple conjugates to the complex, enabling a much brighter signal. Another benefit of the indirect method is access to fluors or channels in which directly conjugated antibodies are unavailable, increasing the flexibility of the assay.
VHH antibodies for flow?
The format of JIR’s AffiniPure-VHH™ secondary antibodies makes them an excellent choice for flow cytometry. Being smaller than conventional antibodies and fab fragments at 15kDa, they can decorate the primary antibody with high efficacy. In addition, they lack an Fc domain, limiting potential effector activation and internalization. Finally, their polyclonality ensures heterogeneous binding of the primary antibody, maximizing detection across the whole molecule to ensure optimal signal.
Experimental set-up
Human white blood cells were blocked in 5% normal alpaca serum (028-000-001) and then incubated with Anti-CD3 mouse antibody (BD #555330) at a 1:5 dilution as recommended by manufacturers’ instructions.
This was then followed by the secondary antibody step using Alexa Fluor® 488 AffiniPure-VHH™ Fragment Alpaca Anti-Mouse IgG (H+L) (min X Bov, Hu, Rb Sr Prot)
(615-544-214) at a 1:200 dilution. A control using the secondary antibody only at 1:200 was also performed.
Understanding single-parameter histograms
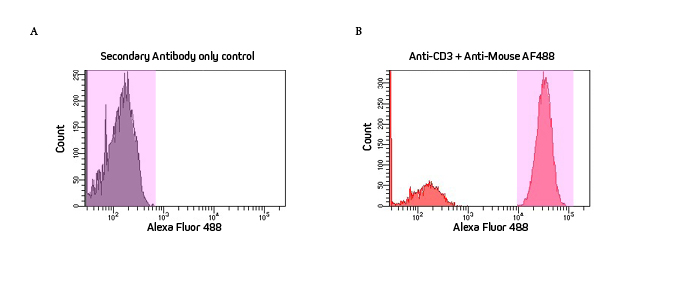
Flow cytometry histograms visually depict the incidents where signal is detected. These events, or “counts” measured for that channel, are displayed as frequency (y-axis) and intensity (x-axis). Figure 1 is a single-parameter histogram that shows the signal from the Alexa Fluor® 488 channel (x-axis) which indicates the detection of CD3 on the cells by the antibody complex.
Figure 1A shows the secondary-only control. Highlighted in pink are the high number of counts (Y axis) of low-intensity signal shown by the peak to the left of the histogram, indicating no staining. This indicates no detection of CD3 on the cells and absence of non-specific binding by the secondary antibody to the cells.
Figure 1B, in contrast, shows the red peak to the right of the histogram representing a high number of high-intensity counts. This indicates positive staining and, therefore, cells positive for the CD3 marker detected by the labeling antibody complex.
Jackson ImmunoResearch specializes in producing secondary antibodies for life science applications. We offer a broad range of secondary antibodies in both whole molecules, F(ab’)2, Fab, and NEW AffiniPure-VHH™ fragment antibodies conjugated to a wide range of fluorescent dyes (including Alexa Fluor®, Brilliant Violet™, and Rhodamine Red™) and proteins (including R-PE, APC, and PerCP), many of which have been literature-cited for flow cytometry. To streamline panel design, researchers can access our Spectra Viewer, powered by FluoroFinder, to compare excitation and emission spectra and determine fluorophore compatibility.
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



