The secondary antibody detects the primary antibody, typically conjugated to a reporter molecule it enables the visualization of the protein(s) of interest. Part 6 of our Western blotting guide details hints, tips and best practices for handling your secondary antibodies for Western blotting.
Overview
Following incubation with the primary antibody and thorough washing, the membrane is incubated with the secondary antibody. The secondary antibody binds the primary antibody and is typically conjugated to a reporter molecule which enables the visualization of the protein(s) of interest.
Advantages of Indirect Detection
Western blotting is typically performed indirectly, using a conjugated secondary antibody instead of a directly conjugated primary antibody. Indirect detection affords greater assay sensitivity and reagent flexibility. Multiple secondary antibodies can bind each primary antibody, and each secondary antibody can bring multiple reporter molecules to the antibody complex, amplifying the signal from each primary antibody.
Indirect detection preserves the affinity of the primary antibody by eliminating the possibility of conjugation interfering with its antigen-binding sites (paratopes), preventing compromised detection of the target molecule antigens.
Directly conjugated primary antibodies are often of limited availability, with a poor range of species or conjugate offered commercially. Greater choice is possible using indirect detection. Secondary antibodies are readily available conjugated to a wide range of reporter molecules, from enzymes, fluorescent dyes and proteins, and to amplification molecules such as biotin.
Indirect detection also enables one secondary antibody to be used with a variety of primary antibodies, which may present better value than purchasing multiple direct conjugates.
Considerations for Choosing a Secondary Antibody – Host Species
Compatibility of Host Species
If using multiple secondary antibodies, for example probing for two primary antibodies, choose the same host species to minimize any cross-reactivity. If this is not possible, then choose secondary antibodies that are cross-adsorbed against the host species of the other secondary antibodies in the experiment.
Cross-Reacting Species
Although not common in western blot, some applications require secondary antibodies that are adsorbed against other species to minimize recognition of endogenous immunoglobulins (such as probing mouse tissue lysate with an anti-rat primary antibody)
or other primary antibodies. Therefore, choose a secondary antibody host that is available with the appropriate cross-adsorptions for the intended application.
Experience or Preference
The choice of host species often comes down to either personal preference or previous experimental validation. There appears to be little difference in quality between secondary antibodies raised in different host species, merely that some species are more appropriate for large-scale production.
Specificity
It is important to pick a secondary antibody with the correct specificity. Although whole IgG (H+L) is suitable for most immunoassays, other specialist specificities allow more complicated experiments to be designed to remove potential interference from unrequired detection.
Anti-IgG (H+L)
These antibodies react with both the heavy and light chains of the IgG molecule, i.e., with both the Fc and F(ab’)2 / Fab regions of IgG (Figure 1). Anti-IgG (H+L) antibodies also react with other immunoglobulin classes (IgM, IgA, IgD, IgE) and subclasses since they all share the same light chains (either kappa or lambda).
Anti-IgG (H+L) antibodies have broader epitope recognition than anti-fragment specific antibodies. They are also suggested for all general immunodetection procedures.
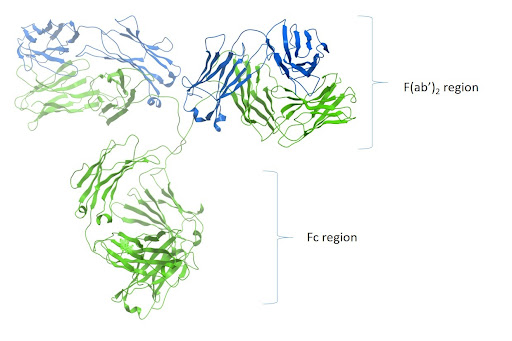
Figure 1: Ribbon diagram of IgG and F(ab’)2 and Fc region
Isotype Specificity: Fc Specific Antibodies
Most primary antibodies are of the IgG isotype. Other classes, such as IgM and IgA, are sometimes used depending on how the antibody has been generated. Secondary antibodies which are specific to the Fc domain of the immunoglobulin will be specific to the isotype of that particular Fc domain.
Therefore, an Fcγ specific antibody will be specific to IgG, whereas an antibody specific to Fcα will detect IgA specifically, and Fcμ will detect IgM.
Pan-specific antibodies are also available, which can be used to detect two or more isotypes. Depending on your application, this may be useful. Choose a secondary antibody that is specific to the isotype of the primary antibody.
Subclass Specificity
IgG-specific antibodies will detect all gamma isotype immunoglobulins due to the similarity of their homologous Fcγ domains. Depending on the mouse strain used to generate the primary antibody, the subclass of the immunoglobulin could be 1 of 5. It may be the case that you have two mouse antibodies of different subclasses targeting two proteins in the same experiment. To differentiate between the two primary antibodies, you would require subclass-specific antibodies.
Mice express four of the five available IgG subclasses making up their IgG isotype. They typically produce IgG1, IgG2b and IgG3, and depending on their strain, they will also express either IgG2a or IgG2c.¹ Jackson ImmunoResearch Anti-Mouse IgG subclass specific antibodies offer specificity to the five individual mouse IgG subclasses. These highly specific antibodies are designed to distinguish between two or more different subclasses of mouse IgG in multiplex experiments or for mouse IgG subclass determination. They have been adsorbed against human, bovine and rabbit serum proteins to minimize cross-reactivity with tissue immunoglobulins, adherent bovine IgG on cultured cells, and rabbit primary antibodies. Subclass-specific antibodies provide exquisite discrimination between the subclasses.
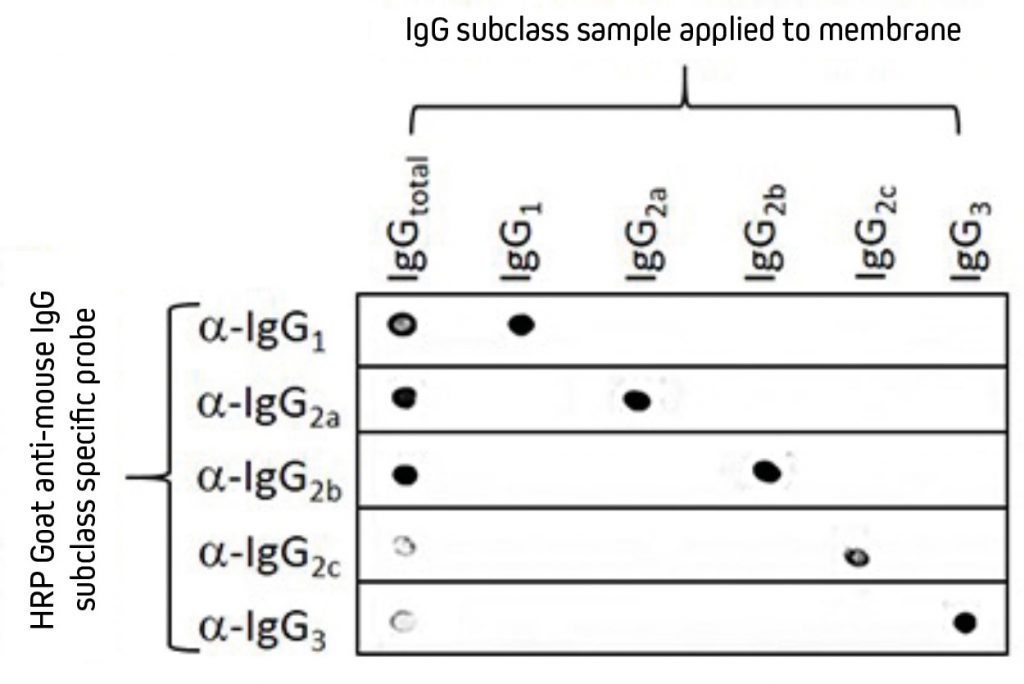 Separate nitrocellulose strips (rows) received 100 ng “dots’’ of mouse IgG and each subclass and then were blocked with 5% (w/v) BSA in PBST. After probing with HRP-conjugated Goat Anti-Mouse subclass-specific antibodies, the strips were developed with TMBM substrate. The grid of positive signals shows the specificity of each subclass-directed antibody. Some subclasses are poorly represented in a total IgG pool and thus give a weak signal (IgG total vs. Anti-IgG2c and -IgG3). HRP-conjugates used for probing were 115-035-205 (Anti-Mouse IgG1), 115-035-206 (Anti-Mouse IgG2a), 115-035-207 (Anti-Mouse IgG2b), 115-035-208 (Anti-Mouse IgG2c) and 115-035-209 (Anti-Mouse IgG3). |
CautionAnti-IgG, Fcγ fragment specific antibodies do not react equally with all monoclonal primary antibodies. For an anti-mouse IgG, Fcγ fragment specific antibody with balanced reactivity to four subclasses of IgG, select Goat Anti-Mouse IgG (subclasses 1+2a+2b+3), Fcγ fragment specific (min X Hu, Bov, Rb Sr Prot). Additionally, subclass-specific antibodies are not necessary for general detection of mouse monoclonal antibodies in single-labeling experiments or multiple-labeling experiments involving one mouse monoclonal and primary antibodies from other species. |
Anti-IgG: Light Chain Specific
These antibodies react with the light chains shared by IgG and the other immunoglobulins. They were developed to facilitate the detection of proteins around 50 kDa on western blots after immunoprecipitation (IP) and do not react with IgG heavy chains.
| Western Blotting after Immunoprecipitation
For researchers who perform western blotting following immunoprecipitation, antibodies specific for light chains or Fc fragments allow unobstructed detection of antigens in the 50 kDa or 25 kDa ranges, respectively. Anti-Light Chain Specific AntibodiesWhen labeled secondary antibodies specific for both heavy and light chains of IgG (such as anti-IgG (H+L)) are used to detect protein bands on western blots following immunoprecipitation (IP), two bands appear (see Figure 3) corresponding to the heavy (50 kDa) and light (25 kDa) chains of the precipitated primary antibody. These bands usually obscure the detection of any protein of interest with a molecular weight near 50 kDa or 25 kDa. However, when labeled anti-IgG, Light Chain Specific antibodies are used for detection; they bind only to the light chain band on the blot (Figure 3) and to light chains on the native primary antibodies used for detection. Therefore, a 50 kDa protein may be detected on blots without interference from the heavy chain of the precipitating IgG. Light chain-specific antibodies are available directed against goat, mouse, rabbit, rat and sheep. They have been adsorbed to minimize cross-reactivity with immunoglobulins from many other species, which also may be present on blots. 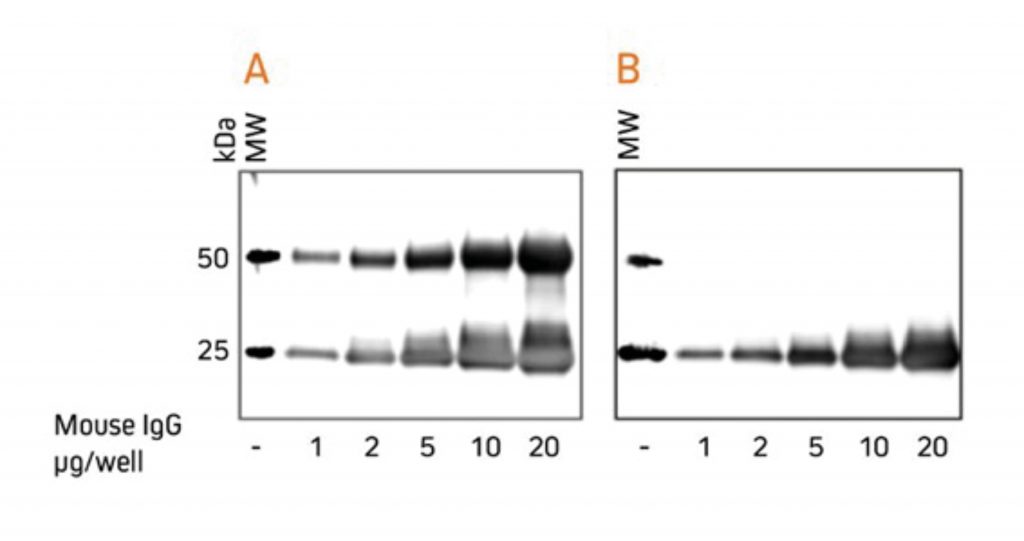 Gels were loaded with reduced and denatured Mouse IgG, whole molecules. After SDS-PAGE and transfer to nitrocellulose, blots were blocked with BSA (10% w/v). After incubation with the secondary antibody, blots were developed with ECL substrate. Blots were imaged simultaneously, with auto exposure time based on bright bands. A: The gel was probed using HRP-conjugated Goat Anti-Mouse IgG (H+L) (115‑035‑003), revealing bands corresponding to both heavy chains (50 kDa) and light chains (25 kDa). B: The gel was probed using HRP-conjugated Goat Anti-Mouse IgG, light chain specific (115‑035‑174), revealing only the 25 kDa band corresponding to Ig light chains. The IP antibody heavy chain is not detected, allowing visualization of the protein of interest near 50 kDa. Heavy Chain Specific Detection on Western Blots after IP with Anti-Fc Specific AntibodiesAnti-IgG, Fc fragment specific antibodies may be used to detect native IgG primary antibodies without binding to the 25 kDa band of reduced and denatured IgG light chains on western blots. Using these antibodies allows clear detection of a 25 kDa analyte without interference from the light chains of an IP antibody. However, this detection is complicated by the appearance of degraded heavy chain antibody at 25 kDa, see Figure 3 panel A. To avoid signal from a degraded heavy chain at 25 kDa, block with monovalent Fab fragment anti-Fc (FabuLight™), see Figure 4, panels B and C. The extreme sensitivity of western blotting requires high concentrations of the blocking reagent. 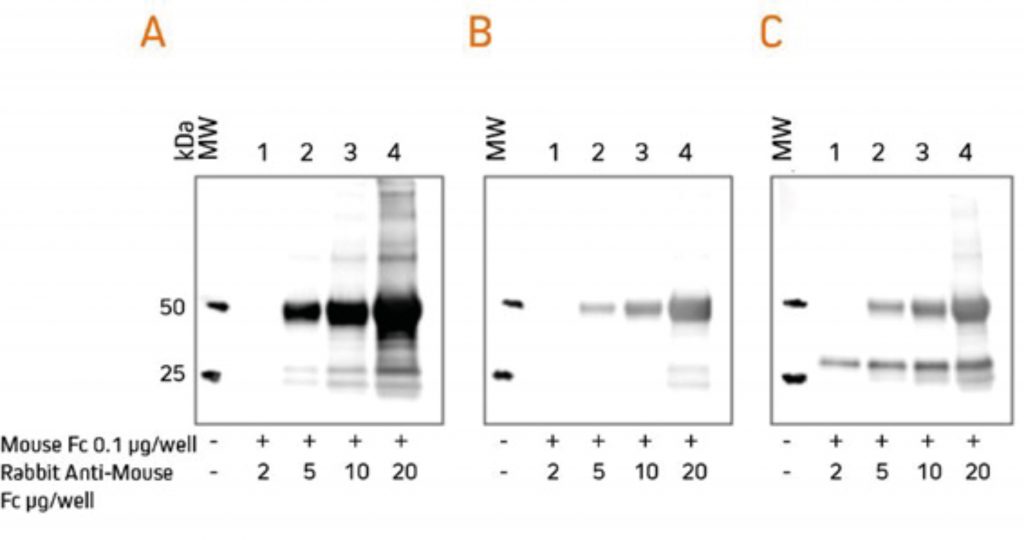 Rabbit Anti-Mouse IgG, Fcγ fragment specific (315-005-008) was mixed with ChromPure™ Mouse Fc (015-000-008) to simulate immunoprecipitation (IP). Three identical gels were run, lanes loaded with denatured and reduced: Lane 1: 2 μg Rabbit Anti-Mouse Fc + 0.1 μg Mouse Fc Lane 2: 5 μg Rabbit Anti-Mouse Fc + 0.1 μg Mouse Fc Lane 3: 10 μg Rabbit Anti-Mouse Fc + 0.1 μg Mouse Fc Lane 4: 20 μg Rabbit Anti-Mouse Fc + 0.1 μg Mouse Fc After SDS-PAGE and transfer to nitrocellulose, blots were blocked with BSA (10% w/v). Subsequent incubations are shown below. After incubation with the secondary antibody, blots were developed with ECL substrate. Blots were imaged simultaneously, with auto exposure time based on bright bands. A: No FabuLight block
The secondary antibody detects the IP antibody heavy chain (HC) at 50 kDa and degraded HC at 25 kDa. B: FabuLight block: Fab Goat Anti-Rabbit IgG, Fc (111-007-008), 200 μg/ml
The signal from IP antibody HC is greatly reduced. At lower loading amounts (2, 5 and 10 μg), the degraded HC at 25 kDa is not detectable. C: FabuLight block: Fab Goat Anti-Rabbit IgG, Fc (111-007-008), 200 μg/ml
The secondary antibody detects the primary antibody, revealing a protein of interest at 25 kDa. Note that the protein of interest shows the sharpest band when the amount of IP antibody is low. To block the signal from a mouse IP antibody, use the Fab fragment corresponding to the subclass of the IP antibody. |
Anti-IgG, F(ab’)2 Fragment Specific
These antibodies react with the F(ab’)2 / Fab portion of IgG. They have been tested by ELISA and/or adsorbed against Fc fragments. They are not specific for IgG since they react with light chains, and they therefore also react with other immunoglobulin classes (IgA, IgM, IgD and IgE) and subclasses sharing the same light chains.
They are predominantly used to detect scFvs (Single-chain variable fragment antibodies). IgG F(ab’)2 fragment specific antibodies may be used in western blotting where enhanced sensitivity to the F(ab’)2 antibody is required and may offer advantages over whole molecule specificity
Using secondary antibodies as a negative control to isolate the cause of non-specific signalA secondary antibody-only control can help to identify the cause of non-specific signals, be that unexpected bands or diffuse signals. Block the membrane with an appropriate blocking solution (5% v/v normal serum of the labeled antibody) and incubate the membrane with the secondary antibody for the prescribed duration, and wash and apply the desired visualization method such as ECL substrate or imager.
|
Determining Optimal Working Concentration
The sensitivity of the secondary antibodies depends on the specificity of the primary antibody and the abundance of the protein of interest. By increasing the concentration of the antibodies, the background signal may increase beyond that of the analyte’s signal.
The correct concentration must be determined to achieve a high signal-to-noise ratio so that background signal does not interfere with detection of the target protein and result interpretation.
Poorly expressed proteins or primary antibodies with weak affinity may benefit from sample concentration or immunoprecipitation rather than extended incubations or more concentrated antibody solutions.
HRP conjugates can offer excellent sensitivity when paired with high-quality chemiluminescent substrates such as ECL, enabling reported antibody dilutions between 1:20,000 and 1:100,000. Optimum working dilution should be determined by titration.
Stepwise dilution of each primary antibody and secondary antibody can be performed easily using a dot blot grid whereby concentrations of antigen and detection reagents can be evaluated and limits of detection determined.
Reprobing and Stripping for Additional Target Proteins
Membranes may be stripped to remove the primary and secondary antibody complex and reprobed with a second pair of antibodies to allow the detection of a second target protein.
The membrane is stripped with a low pH buffer such as 1M glycine to remove the primary and secondary antibody complex from the antigen bound to the membrane. The membrane is then blocked and incubated with a new primary antibody, washed and incubated with a compatible secondary antibody and substrate before being reimaged again.
The success of stripping can be highly variable and must be carefully performed to prevent either being too harsh and damaging the antigen. Alternatively, conditions too mild to strip the antibody complex from the membrane may lead to continued detection.
The results of a reprobed blot should only be used for confirmation, not for quantitation. Choosing a second primary antibody raised in a different host to the initial antibody can help improve the specificity of the reprobing. Stripping and reprobing membranes for additional targets are typically applied in chemiluminescent detection, as fluorescent conjugates offer a more reliable method for detecting multiple targets. See Section 8 for more information about multiplex detection.
|
Caution: Secondary antibody diluent buffer Avoid adding proteins such as powdered milk or BSA to antibody diluent solutions to prevent non-specific interactions that may lead to background signal. Caution: Batch-to-Batch Variation It is also important to note that the concentration of polyclonal antibodies in serum may vary between batch-to-batch and animal-to-animal, so the dilution curve should be repeated if a change in the analysis is observed. Choose high-quality JIR Affinipure™ affinity-purified antibodies to ensure batch-to-batch consistency between lots. |
| Our rigorous quality assurance process ensures a standardized concentration of immunoglobulin in each vial with assured minimal cross-reactivity to stated species and confirmed reactivity to the desired target. |
Secondary Antibody Formats
Whole IgG Antibodies
Whole IgG antibodies are generally the only format required for western blotting, consisting of both heavy and light chains making up bivalent Fab arms and Fc domain.
F(ab’)2 Antibodies
F(ab’)2 secondary antibodies have been enzymatically processed to remove the Fc portion. This may be beneficial in some experimental conditions, such as to avoid binding to Fc receptors on live cells or to Protein A or Protein G. However, typically, these considerations offer no advantage to western blotting over whole IgG antibodies.
Fab Fragment Antibodies
Fab fragments are generated by digestion of whole IgG antibodies with papain to remove the entire Fc region and the disulfide bond between the Fab fragments. These antibody fragments can be used to block immunoglobulins derived from the sample material.
Fab fragments specific for the Fc domain can be used in western blotting after immunoprecipitation in combination with Fc-specific antibodies to block the signal from degraded heavy chains. See the above section for more details.
Found Part 6 of our Western blotting guide useful? View part 7 here
References
- Collins, A. (2016). IgG subclass co-expression brings harmony to the quartet model of murine IgG function. https://doi.org/10.1038/icb.2016.65
- Jackson ImmunoResearch Laboratories Inc. (2017). Western Blotting Troubleshooting Guide! https://www.jacksonimmuno.com/secondary-antibody-resource/technical-tips/western-blot-trouble-shooting/
- Lewis M. (2018). Cross-Adsorbed Secondary Antibodies and Cross-Reactivity. Jackson ImmunoResearch Laboratories Inc.https://www.jacksonimmuno.com/secondary-antibody-resource/technical-tips/cross-adsorbed-and-cross-reactivity/
| Learn more: | Do more: |
|---|---|
| Colorimetric western blotting | Spectra Viewer |
| Chemiluminescence western blotting | Antibodies for signal enhancement |
| Fluorescent western blotting | |



