
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Flow cytometry is a technique regularly used for screening and quantifying proteins expressed on the cell surface. The GFP tag is frequently used in flow cytometry as a reporter molecule to track expression of fusion proteins. Learn how Jackson ImmunoResearch's two Anti-GFP antibody formats, a whole molecule raised in Rabbit, and AffiniPure-VHH® Fragment Alpaca Anti-GFP, a polyclonal NANOBODY®, can detect a GFP fusion protein expressed on yeast cells by flow cytometry.

GFP is a 27kDa protein that emits green fluorescence when exposed to UV (ultraviolet) light. One of its derivatives, among others, is enhanced GFP (EGFP), which modifies the original jellyfish GFP (avGFP), introducing two mutations (F64L, S65T) to improve brightness (Tsien, 1998).
EGFP is regularly used in fusion proteins as both an expression marker and as a reporter molecule. This is because it expresses as a functional, mature protein in most cell systems, doesn't require additional cofactors, is easily visualized with common fluorescence microscopy set-ups, and is well-validated, making it reliable as a fusion protein. EGFP is regularly used as a reporter molecule to follow the gene expression in transiently transfected cells (Subramanian et al.,1996) and stable cell lines.
Although EGFP is a robust and tractable molecule, sample processing or experimental limitations can hinder its use in some conditions. We cover this below and introduce Anti-GFP antibodies as a solution to enable the use of GFP tags and bypass its limitations when conditions are suboptimal.
Autofluorescence is a type of intrinsic fluorescence that occurs due to the presence of cellular components. This fluorescence signal generates background that can interfere with the detection and interpretation of genuine signals from target proteins. Autofluorescence may occur across the spectrum but typically emits between 350 and 550 nm, which overlaps with the emission profile of GFP; this can be problematic because the GFP signal cannot be distinguished from the background autofluorescence. Anti-GFP antibodies conjugated with a fluorophore that emits at a different, often longer, wavelength can circumvent these limitations by shifting GFP emission to another channel, allowing it to be distinguished from autofluorescence.
GFP, like many proteins, is sensitive to environmental conditions. For GFP to fluoresce, it must form a fully folded beta-barrel structure. This is then followed by an intramolecular reaction that requires oxygen to generate the chromophore (Tsien, 1998). The consequence of this requirement is that GFP is non-fluorescent under anaerobic conditions. Another limitation of GFP that prevents its functionality is its sensitivity to acid. GFP's chromophore can only absorb and emit light in its protonated state (Tsien, 1998). Therefore, at pHs below its pKa (<pH 6.0), where the chromophore is present in its deprotonated state, the efficiency of the protein to fluoresce is reduced.
Another issue with GFP, when expressed as a trans-protein under the promoter of a gene of interest, is that it may not be tethered within the cell and can diffuse within the cytosol. This may cause problems with subsequent immunostaining, where GFP can be lost during wash steps (Chalfie and Kain, 2005; Morris et al., 2010). The free movement of GFP can be prevented by fixing the tissue/samples. However, this process, particularly FFPE, can functionally alter the GFP protein, weakening and destroying the protein's ability to fluoresce (Kusser et al., 2003).
JIR Anti-GFP antibodies offer robust detection of GFP, recognizing GFP in either native or fixed/denatured conditions as long as the protein is not degraded, enabling the GFP tag to be used in circumstances where the protein would not be functional. The polyclonal format of JIR Rabbit and Alpaca VHH Anti-GFP enables the binding of multiple antibodies to the target, thereby bringing additional fluorescent molecules and enhancing the GFP signal beyond the output associated with GFP alone.
Another limitation of GFP relevant to flow cytometry is the choice of fluorescent reporters. Often, flow cytometry uses fluorescent proteins such as Phycoerythrin, which provide robust and reliable signal. However, PE and GFP have overlapping spectral characteristics, making it difficult to excite the molecules separately and sufficiently deconvolute their signals from each other. Using an Anti-GFP antibody conjugated to a fluorophore with an emission profile in a longer wavelength, such as Alexa Fluor® 647, enables signals to be resolved without the need for spectral unmixing, which often requires additional or expensive equipment or additional data compensation. Conjugated Anti-GFP antibodies can also add versatility to experiments restricted by equipment limitations, for example, when a laser line or channel is occupied by another target. Having the option to use different channels can be useful when labeling multiple targets.
Jackson ImmunoResearch offers AffiniPure® Rabbit Anti-GFP and AffiniPure®-VHH Fragment Alpaca anti-GFP. Both are affinity-purified polyclonal antibodies specific for GFP and its derivatives. The antibody's polyclonal format enables spectacular signal amplification of expressed GFP. Learn how Jackson ImmunoResearch Anti-GFP antibodies can be used to detect a GFP fusion protein expressed on yeast cells by flow cytometry in the following case studies.
Recombinant proteins can be expressed in various systems, including bacterial, insect, mammalian, and fungal/yeast. Recombinant proteins are often expressed as fusions with reporter molecules, such as GFP, to facilitate their detection, purification, or characterization of interactions using techniques like Förster resonance energy transfer (FRET). Flow cytometry is a useful technique for screening and quantifying expression, particularly when proteins are expressed on the surface of a cell. Here, we investigate the expression of EGFP fused to Aga2p, a yeast mating protein. Aga2p will form a heterodimer complex with Aga1p, resulting in EGFP display on yeast surface.
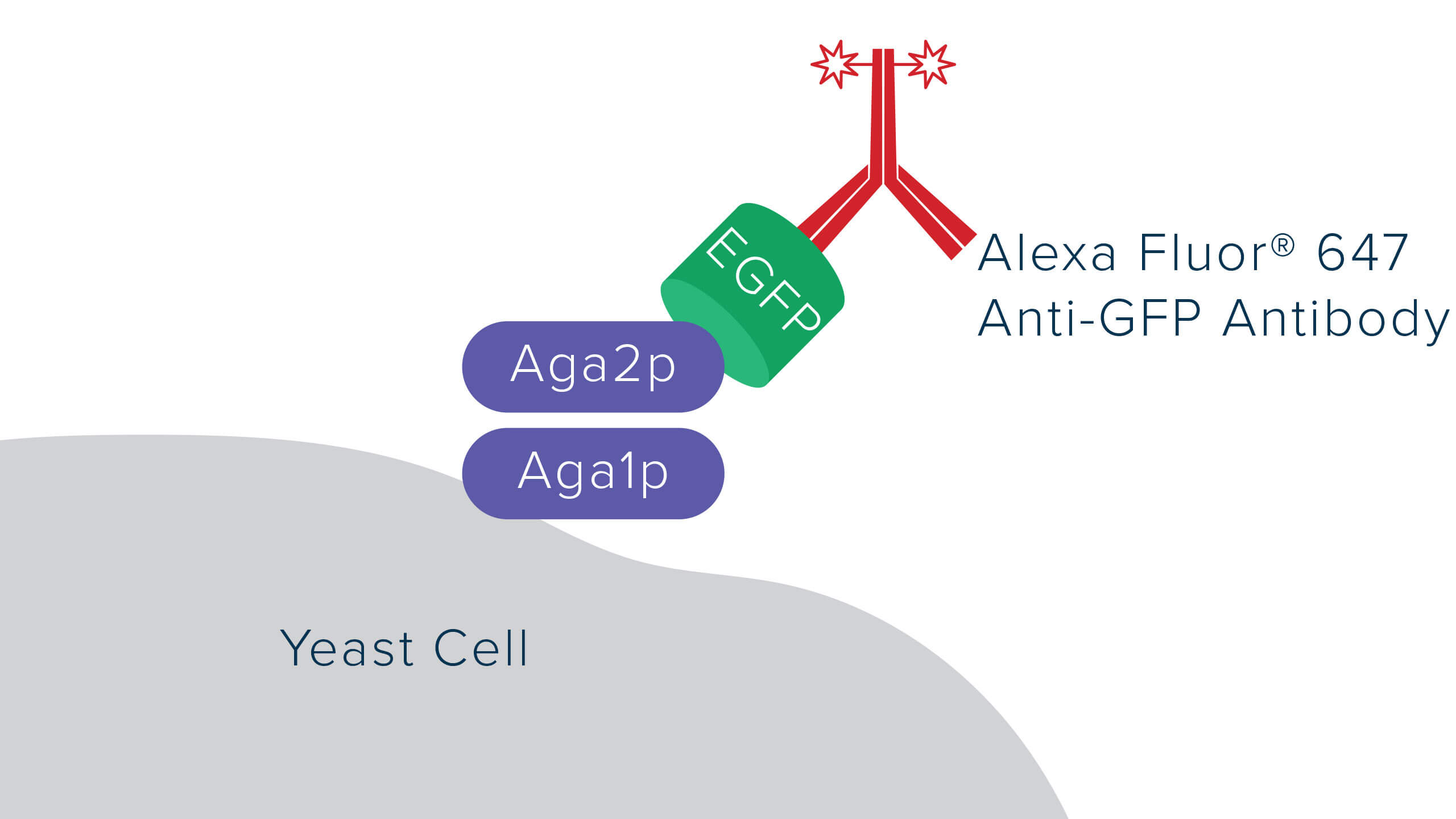
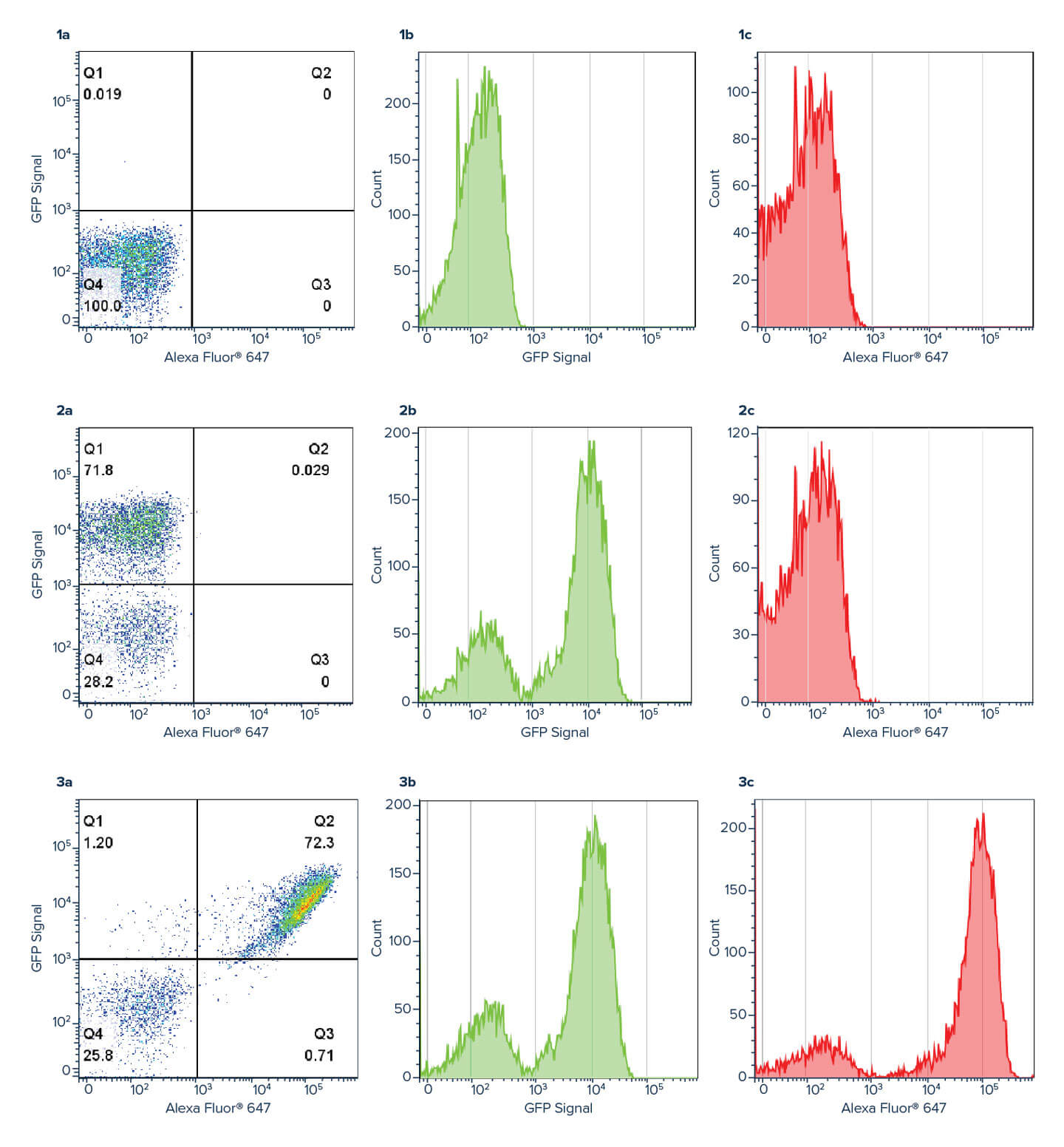
Using an Anti-GFP antibody allows the GFP signal to be amplified, with a log shift in brightness observed between the green GFP channel (Figure 3b) and the red Alexa Fluor® 647 Anti-GFP channel (figure 3c). This amplification can help improve the deconvolution of data where channels may overlap, or the signal is indistinguishable from background.
EBY100 yeast (ATCC, MYA-4941) cells were transformed with a plasmid containing Aga2p-EGFP fusion gene using Frozen-EZ yeast transformation II kit (Zymo Research). Yeast cells were grown in SDCAA media (Teknova) lacking tryptophan as a selection marker at 30°C and 200 r.p.m. Protein expression was induced with a 1:10 dilution of yeast grown in SDCAA into SGCAA media (Teknova) overnight at 30°C. The next day, 200 uL of cells were washed with PBS + 0.2% BSA and pelleted before staining with Alexa Fluor® 647 Rabbit Anti-GFP for one hour at room temperature (RT), followed by a wash step before resuspension in PBS + 0.2% BSA. Flow cytometry was performed using BD FACSCelesta and BD FACSDiva software (BD Biosciences). Analysis was performed using FlowJo software. Method adapted from Butler et al., 2022.
Flow cytometry is a valuable technique for screening and quantifying protein expression, particularly when proteins are located on the cell surface. Flow cytometry frequently uses the GFP tag as a reporter molecule to track protein expression. VHH domain or popularly known as a NANOBODY®, has become an important tool in flow cytometry. Due to its monovalent nature, the VHH fragment antibody enables the pre-mixing of primary and secondary antibodies, thereby reducing the time spent on sequential labeling, which could have an inverse effect on cell viability. Jackson ImmunoResearch AffiniPure-VHH® Fragment Alpaca Anti-GFP antibodies can be used in conjunction with VHH or other monovalent secondary antibodies to multi-label target proteins on cell surface. Here we demonstrate the detection of EGFP on yeast cell surface with our VHH anti-GFP by flow cytometry.
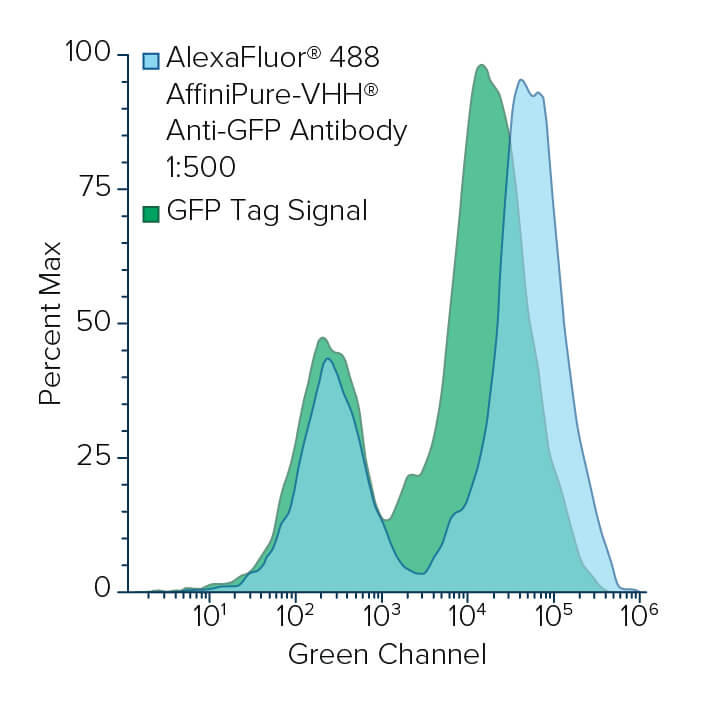
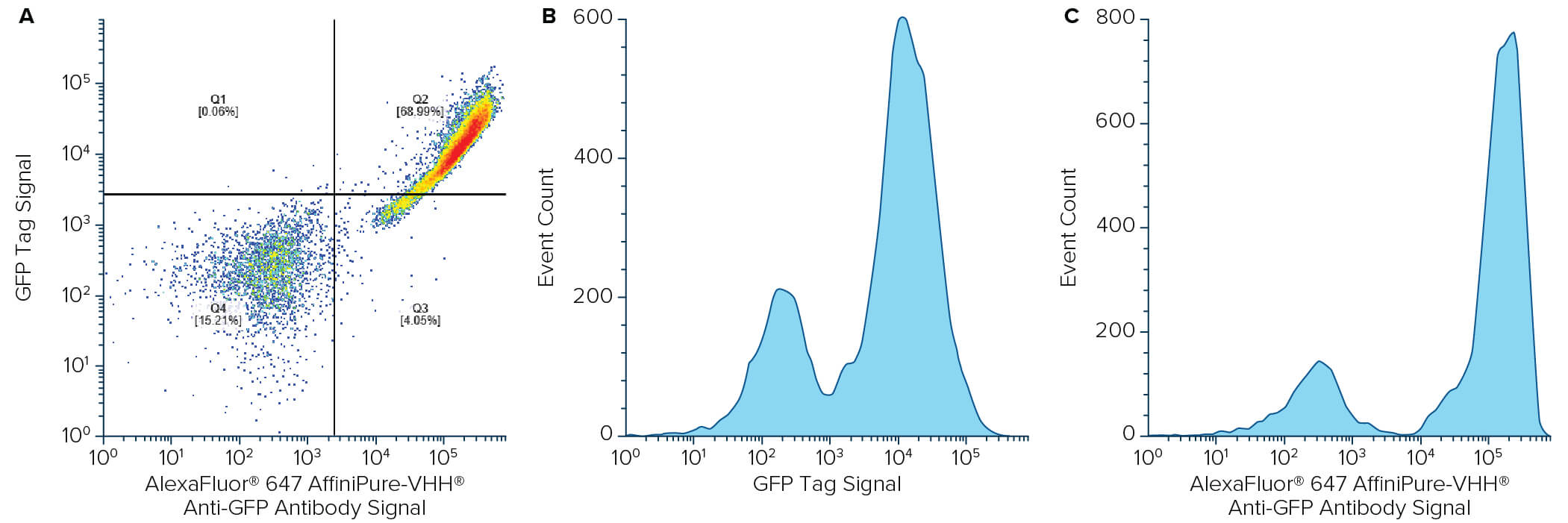
EBY100 yeast (ATCC, MYA-4941) cells were transformed with a plasmid containing Aga2p-EGFP fusion gene using Frozen-EZ yeast transformation II kit (Zymo Research). Yeast cells were grown in SDCAA media (Teknova) lacking tryptophan as a selection marker at 30°C and 200 r.p.m. Protein expression was induced with a 1:10 dilution of yeast grown in SDCAA into SGCAA media (Teknova) overnight at 30°C. The next day, 200 μL of cells were washed with PBS containing 0.2% BSA and pelleted before staining with Alexa Fluor® 488 or 647-conjugated AffiniPure-VHH® Fragment Alpaca Anti-GFP for one hour at room temperature (RT), followed by a wash step and resuspension in PBS containing 0.2% BSA. Flow cytometry was performed using BD FACSCelesta and BD FACSDiva software (BD Biosciences). Analysis was performed using FlowJo software. Method adapted from Butler et al., 2022.