"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
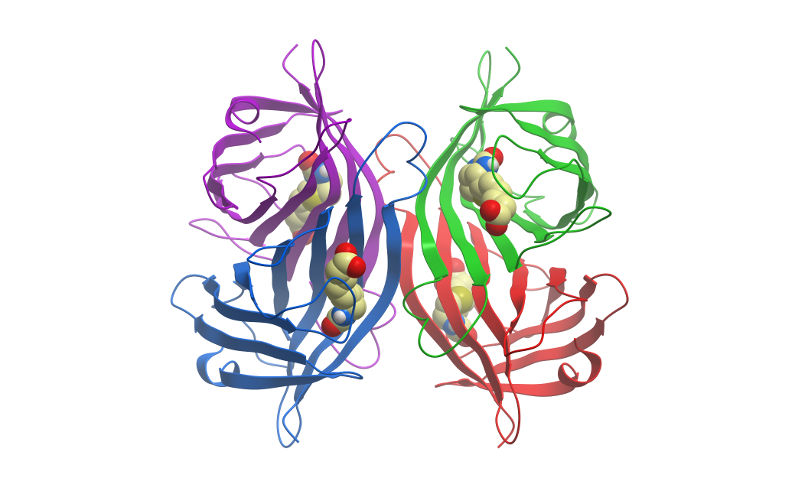
Streptavidin is a tetrameric bacterial protein isolated from Streptomyces avidinii providing 4 high-affinity biotin binding sites (Figure 1). Each monomer is roughly 13kDa, forming an approximately 52 to 55 kDa homo tetramer in its native state. Comparisons of apo and liganded streptavidin crystal structures by Weber et al. (1989) showed that affinity is conferred by multiple hydrogen bonds and van der Waals interactions, which in conjunction with polypeptide loops, confine the biotin in streptavidin’s interior. The result is one of the strongest non-covalent bonds found in nature, with a femtomolar dissociation constant (Kd ~10-15). Unlike egg-white avidin, which has a net positive charge at neutral pH and contains about 7% carbohydrate, streptavidin has almost no net charge at neutral pH, does not contain carbohydrate, and exhibits lower non-specific background.
Because of its binding characteristics, streptavidin is commonly employed for immunotechniques requiring signal amplification using biotinylated reagents.
Jackson ImmunoResearch streptavidin conjugates are recommended for use with Biotin-SP-conjugated affinity-purified secondary antibodies and ChromPure™ proteins, as well as with any biotinylated primary or secondary antibody, or oligonucleotide.
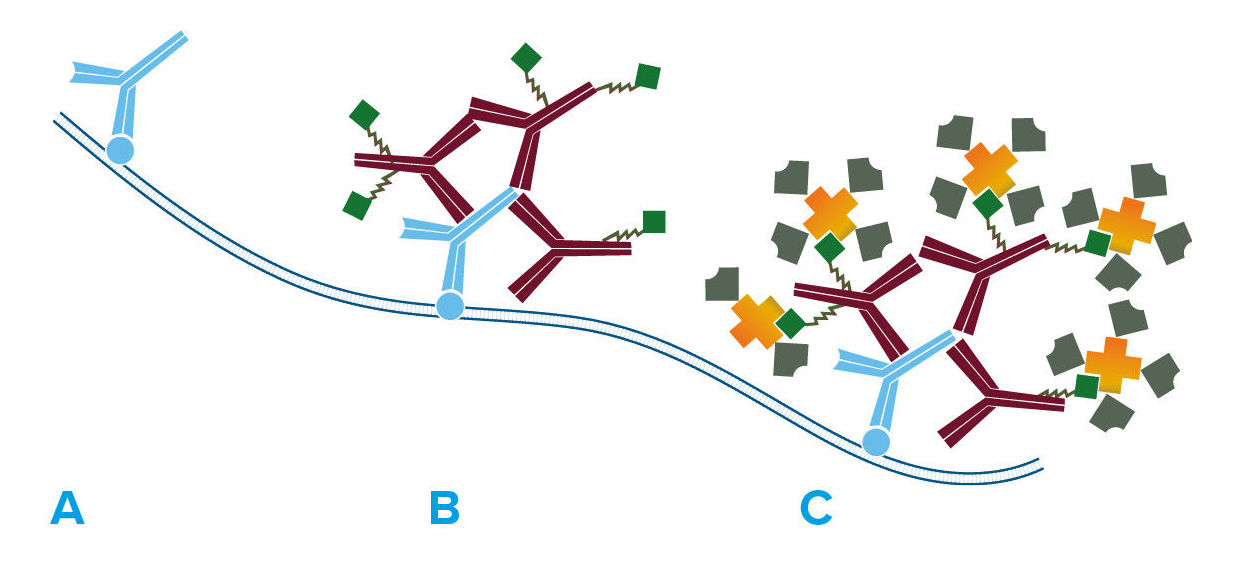
A number of signal amplification techniques are possible with Jackson ImmunoResearch secondary antibodies. Streptavidin is offered for signal enhancement (Figure 2) as a superior technique to the avidin-biotin-HRP complex (ABC) method. Compared with the ABC method, HRP-conjugated streptavidin is more stable, gives less background, and is more sensitive as reported by Shi et al. (1988) and Milde et al. (1989). The increased sensitivity may be due to enhanced tissue penetration and less steric hindrance, since nominal molecular weights for all components of HRP conjugated Streptavidin total less than 200 kDa, considerably lower than the weight of ABC.
Jackson ImmunoResearch offers a comprehensive list of fluorophores and enzymes conjugated to streptavidin for use in enzyme immunoassays, immunohistochemistry, flow cytometry, in situ hybridization, and immunoblotting procedures. Most streptavidin products are freeze-dried in buffer containing stabilizers and preservative. Exceptions are unconjugated streptavidin (freeze-dried from a sodium chloride solution), HRP-conjugated streptavidin (freeze-dried without preservative) and alkaline phosphatase-conjugated streptavidin (sterile-filtered liquid with preservative).