
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Green Fluorescent Protein (GFP) is a commonly used protein tag for generating fusion proteins that can be expressed in various prokaryotic and eukaryotic systems. The GFP tag is a useful reporter molecule because it does not require exogenous substrates or cofactors to generate fluorescence. GFP is employed in numerous applications, including quantification of gene expression, protein localization within living organisms, studying protein interactions, and as a biosensor. The addition of an Anti-GFP antibody may be used to improve experimental performance and overcome limitations that use of the tag itself may incur.
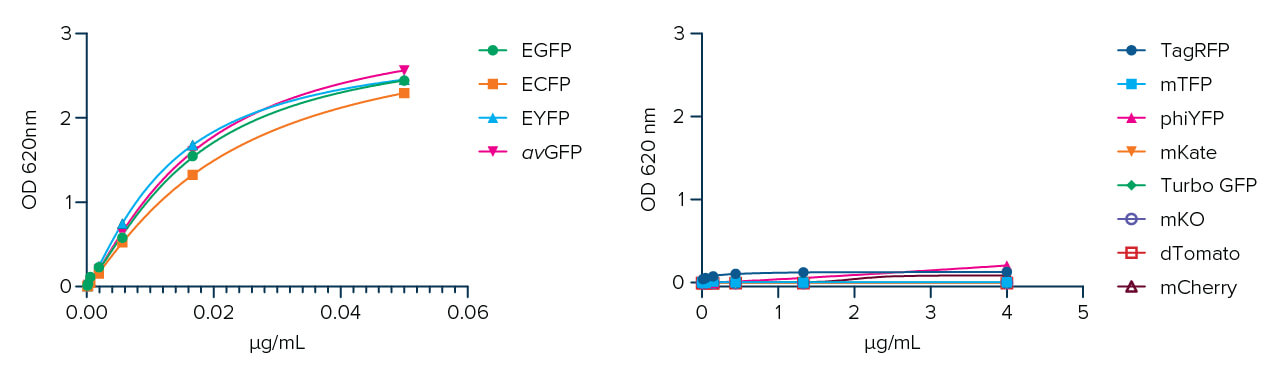
Jackson ImmunoResearch AffiniPure-VHH® Anti-GFP is a polyclonal VHH fragment from an Alpaca host. It can be used to detect Aequorea victoria Green Fluorescent Protein (avGFP) and its derivatives, including EGFP, ECFP, and EYFP (Fig. 1).
Camelid species such as Alpaca and Llama produce a unique class of antibodies composed only of heavy chains. The antigen-binding fragments (Fab) are also referred to as Variable Heavy-Chain only fragment antibodies (VHH Fragments), or NANOBODIES®. AffiniPure-VHH® Anti-GFP antibodies are comprised of this exciting, novel antibody format, which offers outstanding specificity and penetration, and are a fantastic solution for high-quality and high-resolution imaging.
VHH fragment antibodies are a 10th of the size of conventional antibodies. The small diameter of the NANOBODY® format brings the fluorescent probe and the antigen into close enough proximity. This is necessary for accurate localization, allowing the generation of higher-resolution images (Pleiner et al 2015, Carrington et al 2019, Shrestha et al 2015, Croup et al 2019, Gormal et al 2020, Lelek et al 2021). Consequently, they have been readily adopted as powerful tools for confocal and high-resolution imaging, as well as detection reagents (De Groeve et al., 2010; Pleiner et al., 2015; Barakat et al., 2022; Erreni et al., 2020).
NANOBODIES® show excellent tissue penetration due to their small size (Chakravarty et al., 2014; Debie et al., 2020; Hernández et al., 2019), which allows them to move freely through the tissue because of a lower diffusion coefficient than conventional antibodies (Jovčevska and Muyldermans, 2019). The absence of a fragment crystallizable (Fc) further enhances the clearance of the molecule from tissues, making it an excellent tool for diagnostic imaging techniques such as Immuno-PET (Krasniqi et al., 2018).
NANOBODIES® are suitable for immunostaining live cells because they lack the Fc fragment of conventional immunoglobulins, which are generally only suitable for labeling fixed samples (Lelek et al, 2021).
The AffiniPure-VHH® Anti-GFP Antibody utilizes these unique characteristics, enabling excellent tissue penetration and clearance without the need for extended incubations or detergents, which may damage the sample.
Polyclonal detection reagents continue to offer greater sensitivity by amplifying signal, even from poorly expressing targets. Polyclonal antibodies provide a heterogeneous population of antibodies, each with a distinct paratope, thereby detecting a different portion of the GFP protein. By binding to sites across the entire protein of interest, rather than a limited specific site, region, or sequence, polyclonal AffiniPure-VHH® Anti-GFP antibodies can decorate the whole protein, enabling higher labeling efficiency and a brighter signal compared with a monoclonal nanobody* (Figure 2).
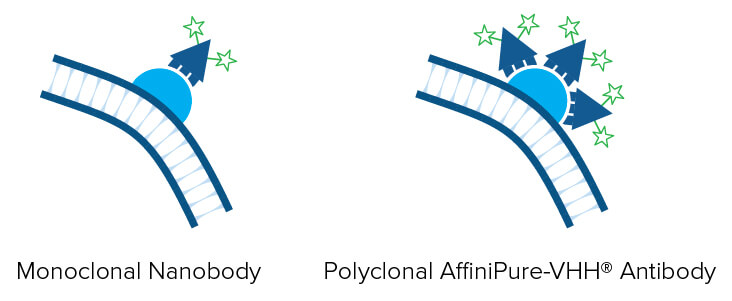
An Anti-GFP antibody can amplify the signal from GFP expressed by cells in IHC or IF experiments. Our AffiniPure-VHH® Anti-GFP antibodies may be used in ELISA, IHC, ICC, IP, Flow cytometry, and WB.
Jackson ImmunoResearch AffiniPure-VHH® Anti-GFP antibodies are available conjugated to a range of Alexa Fluor® dyes. Designed for fluorescence microscopy, these bright dyes, combined with the polyclonal format of the AffiniPure-VHH® Anti-GFP antibodies, deliver bright target signal and spectacular signal amplification.
There are many uses for AffiniPure-VHH® Anti-GFP antibody conjugates, adding versatility to experiments or circumventing the limitations that the GFP tag can present.
| Alexa Fluor® | Excitation Peak (nm) | Emission Peak (nm) |
|---|---|---|
| Alexa Fluor® 488 | 493 | 519 |
| Alexa Fluor® 555 | 552 | 572 |
| Alexa Fluor® 568 | 577 | 602 |
| Alexa Fluor® 594 | 591 | 614 |
| Alexa Fluor® 647 | 651 | 667 |
AffiniPure-VHH® Anti-GFP antibody is also available as a biotin conjugate, facilitating its use in chromogenic IHC staining techniques and amplification protocols such as ABC (Avidin-Biotin-Complex) and LSAB (Labeled Streptavidin-Biotin). Switching to a non-fluorescent detection system enables the use of traditional light microscopy techniques, such as chromogenic IHC.
| Product Description | Product Code | |
|---|---|---|
| Biotin-SP(long spacer) AffiniPure® Rabbit Anti-GFP | 300-065-245 |
Flow cytometry is a valuable technique for screening and quantifying protein expression, particularly when proteins are located on the cell surface. Flow cytometry frequently uses the GFP tag as a reporter molecule to track protein expression. Learn how Jackson ImmunoResearch AffiniPure-VHH® Fragment Alpaca Anti-GFP antibody conjugates can detect a GFP fusion protein expressed on yeast cells by flow cytometry.