
"The anti-human polyclonal capture reagents are some of the most robust reagents I have used in my biophysical and biochemical studies so far. Overall, the anti-Fc reagents regardless of the host or capturing species provides a robust capturing ability with minimum cross-reactivity to other species." Full review.
Rupesh Nanjunda,Rating: 5.0

"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
An enzyme-linked immunosorbent assay (ELISA) is a robust and sensitive technique used to detect and quantify specific proteins in samples that may contain complex mixtures of proteins. Antibodies are used to detect the specific proteins immobilized on the surface of microplate wells. The technique facilitates high volume and fast throughput analysis, ideal for analyzing large numbers of samples.
ELISAs can be performed in a number of ways depending on the sample specifics and the sensitivity required.
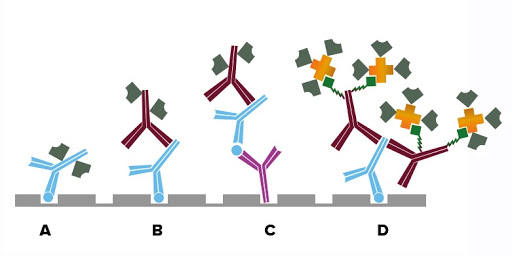
The sample is applied to a protein-binding solid surface, typically a microtiter plate, coating the analyte directly onto the surface, if present (Fig. A). Next, reporter-conjugated primary antibodies specific to the analyte are added. Signal from the directly conjugated reporter molecule is analyzed to provide a quantitative result when used with a standard curve of known concentration. Depending on the sample characteristics this method may have limited sensitivity.
The analyte is coated directly onto a microtiter plate (Fig. B). The sample (biological fluid: serum, saliva, etc.) is added and antigen-specific antibodies, if present, bind to the analyte, forming a complex. Subsequent washing removes non-binding proteins and other components in the sample mixture. A reporter molecule-conjugated secondary antibody is used to detect the bound antigen-specific antibody. Signal generated may be quantified by comparing to a standard curve.
Sandwich ELISAs can be performed both directly or indirectly depending on the level of sensitivity required. There are a variety of methods that use the specificity of antibodies to target different fragments of the antibody allowing greater specificity and versatility.
A primary antibody specific to the antigen (analyte) of interest is immobilized onto a microtiter plate and subsequently captures the analyte from the test sample. A reporter-molecule conjugated primary antibody specific to the antigen is added to complete the sandwich. The signal from the reporter molecule is observed either by adding an enzyme substrate, which results in a colorimetric product or fluorescence, resulting in a readout proportional to the analyte concentration.
A primary antibody specific to the antigen (analyte) of interest is immobilized onto a microtiter plate and subsequently captures the analyte from the test sample (Fig. C). A second primary antibody of a different host species-specific to the antigen is then added to complete the sandwich. A reporter molecule-conjugated secondary antibody binds to the second antigen-specific antibody amplifying signal. The signal from the reporter molecule is observed either by adding an enzyme substrate, which results in a colorimetric product or fluorescence, resulting in a readout proportional to the analyte concentration.
Point of care lateral flow tests for the diagnosis of disease utilize the principles of ELISA.
Read More About Lateral Flow Tests| Step | Direct | Indirect | Indirect Sandwich | Signal Amplification |
|---|---|---|---|---|
| Coat | Analyte | Capture antibody | ||
| Block | We recommend using 5% normal serum of the host species of the detection antibody | |||
| Wash | To remove unbound reagents | |||
| Sample | Conjugated primary antibody | Unconjugated primary | Sample | Sample |
| Wash | To remove unbound reagents | |||
| 2nd | N/A | AP/HRP or fluorophore-conjugated secondary antibody | Biotinylated secondary antibody | |
| Wash | N/A | To remove unbound reagents | ||
| 3rd | N/A | N/A | Detection antibody | Streptavidin |
| Wash | N/A | To remove unbound reagents | ||
| Signal Detection | Colorimetric, chemiluminescent or fluorescent | |||
ELISAs or Enzyme immunoassays (EIA) are frequently used in clinical diagnostic testing; many are validated and available commercially as kits containing all the necessary reagents to perform the test, allowing labs to have access to standardized procedures.
ELISA tests detect immunoglobulins produced as part of an immune or allergic response allowing the diagnosis of infections and allergic diseases, such as food allergy. Alternatively, ELISA can be used to identify causative agents through the detection of the antigen, such as allergens, virus particles, or bacteria, allowing identification of infectious disease.
Typically performed using a polystyrene microtiter plate, the analyte may be coated on the plate, or coated with a capture antibody in the case of a sandwich ELISA. A blocking step using an appropriate serum such as Bovine Serum Albumin (BSA) minimizes the potential for background signal from non-specific interactions between the patient sample and the plate. The patient sample, which may be blood, saliva, or another biological fluid, is introduced to the plate allowing either immunoglobulins or antigens to complex with the capture material. Depending on the format of the assay, signal may be confirmed and quantified by, a reporter molecule-conjugated-primary or secondary antibody, or a biotinylated antigen-specific antibody followed by labeled streptavidin to amplify signal.
Learn About Reagents for Diagnostic TestingBlocking reagents are especially important in ELISA. We recommend using 5% (v/v) normal serum derived from the host species of the labeled antibody to block all unsaturated binding sites on the microplate, although BSA may also be appropriate.
Browse Normal SerumJackson ImmunoResearch alkaline phosphatase (AP) and horseradish peroxidase (HRP) conjugates can be used for colorimetric assays using a chromogenic substrate. For chemiluminescent detection, a luminol based substrate is commonly used with peroxidase conjugates for highly sensitive detection.
Read More About Reporter Enzyme ConjugatesELISAs can also be performed using fluorescent conjugates to allow simultaneous detection of multiple primary antibodies derived from different species. By using labeled secondary antibodies each antigen can be distinguished specifically by the individual fluorescent signal. The detection limit for fluorescent ELISA is typically lower than colorimetric or chemiluminescent detection using a reporter enzyme.
Read More About Fluorophore SelectionSignal enhancement can be achieved using labeled streptavidin to detect a biotinylated antibody (primary or secondary antibody) (Fig. D). Each antibody can present multiple biotin molecules, which are then able to bind to multiple streptavidin molecules. These combined factors mean that multiple probe molecules are available to either catalyze the detection substrate to its end product or generate fluorescent emission, achieving a brighter signal and greater sensitivity.
Read More About Signal EnhancementWe recommend determining appropriate dilution ranges for your experiment, the ranges suggested in the table below are only a guide. We find that higher dilutions may be more appropriate particularly when using the HRP conjugates.
| Product | Conjugate | ELISA |
|---|---|---|
| Whole IgG and F(ab')2 secondary antibodies | Unconjugated | 10-20 µg / ml |
| Whole IgG, F(ab')2 and Fab secondary antibodies | Alexa Fluor® 488, 594, 647, DyLight™ 405, and Cy™3 | 1:100 - 1:800 |
| Whole IgG, F(ab')2 and Fab secondary antibodies | AMCA, BV421™, BV480™, Cy2, FITC, TRITC, RRX, and Texas Red | 1:50 - 1:200 |
| Whole IgG secondary antibodies | Cy™5 | 1:100 - 1:400 |
| Whole IgG and F(ab')2 secondary antibodies | Horseradish Peroxidase | 1:5,000 - 1:100,000 |
| Whole IgG and F(ab')2 secondary antibodies | Alkaline Phosphatase | 1:5,000 - 1:50,000 |
| Whole IgG and F(ab')2 secondary antibodies | Biotin-SP (using enzyme-Conjugated Streptavidin) | 1:20,000 - 1:400,000 |
| Streptavidin | Horseradish Peroxidase | 1-2 µg / ml |
| Streptavidin | Alkaline Phosphatase | 1-2 µg / ml |
| Normal Serum | 5% (v/v) for blocking | |
| ChromPure™ | 10 µg/ml |