
"The anti-human polyclonal capture reagents are some of the most robust reagents I have used in my biophysical and biochemical studies so far. Overall, the anti-Fc reagents regardless of the host or capturing species provides a robust capturing ability with minimum cross-reactivity to other species." Full review.
Rupesh Nanjunda,Rating: 5.0

"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Rapid testing using lateral flow immunoassays (LFIA) has been widely adopted over the past four decades as a means to quickly detect and measure a variety of agents. LFIAs are used in a broad range of applications, from determining the presence of immunoglobulins produced in response to infection or allergy, microorganisms that compromise food safety, substances from genetically modified crops, environmentally hazardous chemicals, and illicit drugs. LFIAs have become a vital component for assuring health and safety in modern life.
The adoption of the LFIA is primarily due to its simplicity of execution and fast generation of results. LFIAs have a dynamic range from mid picomolar to high micromolar (Bishop et al., 2019), and their high specificity can be used to discriminate between strains of pathogens such as SARS-CoV-2 from closely related Coronavirus strains like SARS-CoV-1 and MERS.
This article outlines basic assay formats, critical reagents, and the material components of a typical LFIA. It also illustrates the development of a simple test for human antibodies by LFIA.

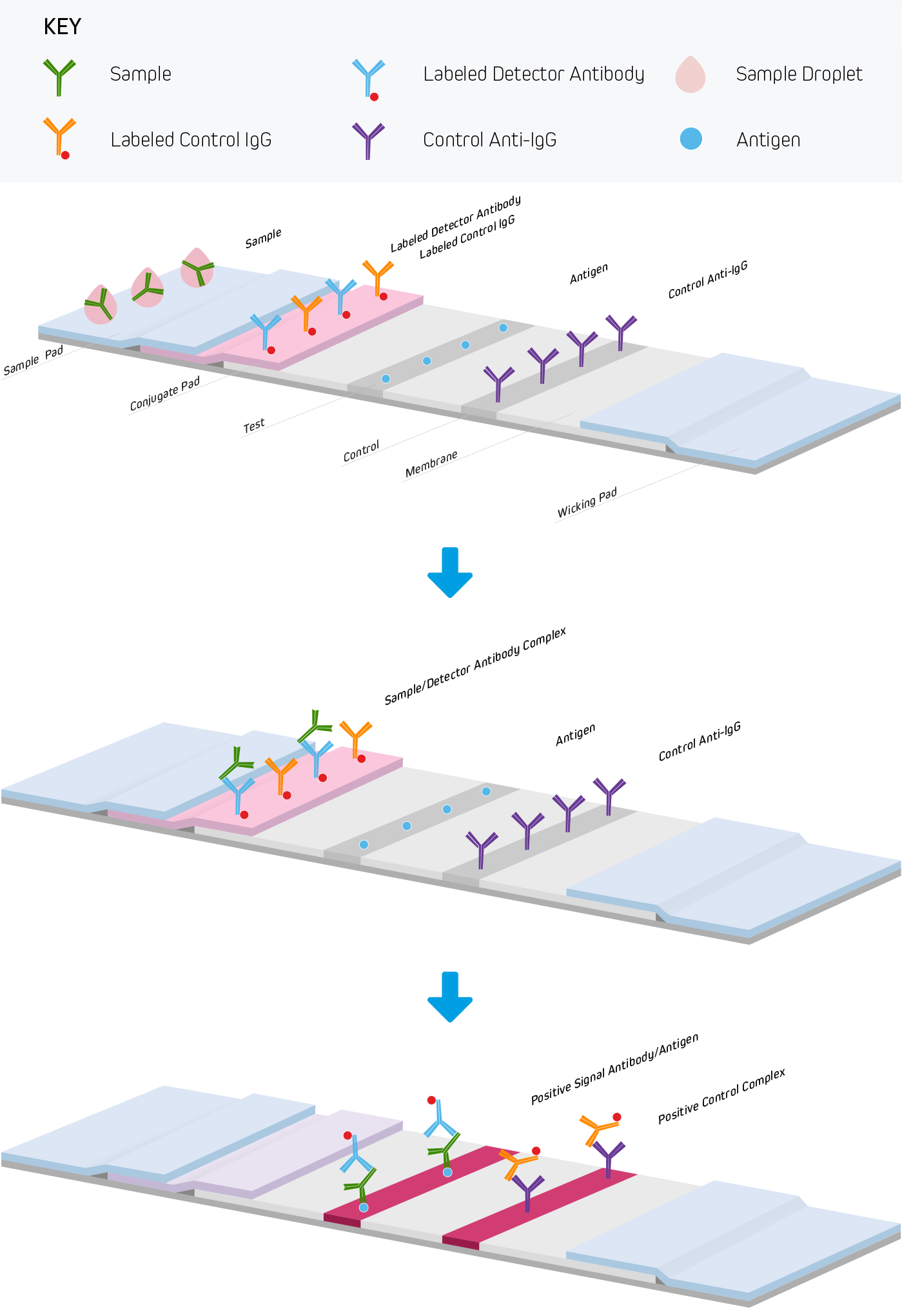
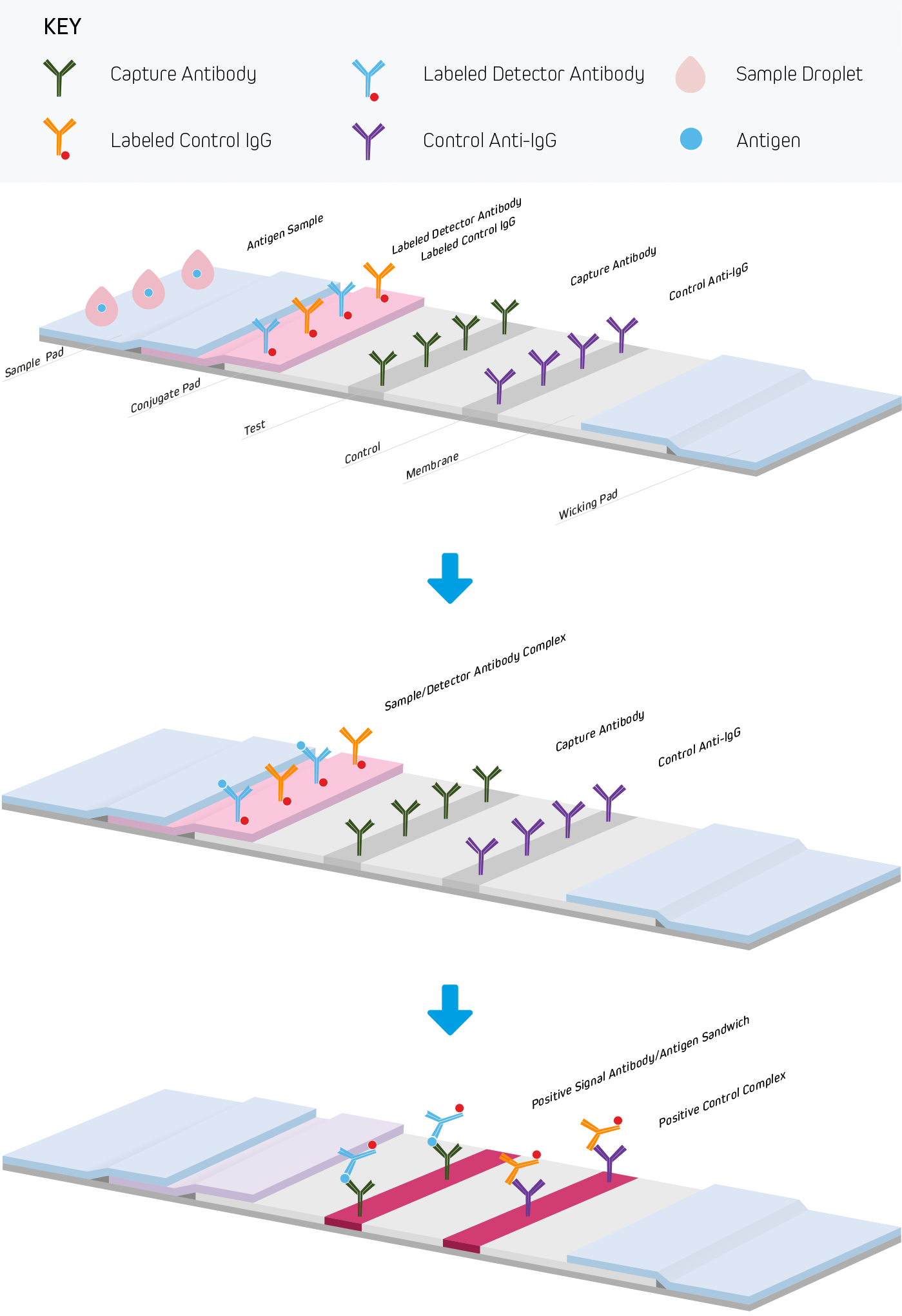
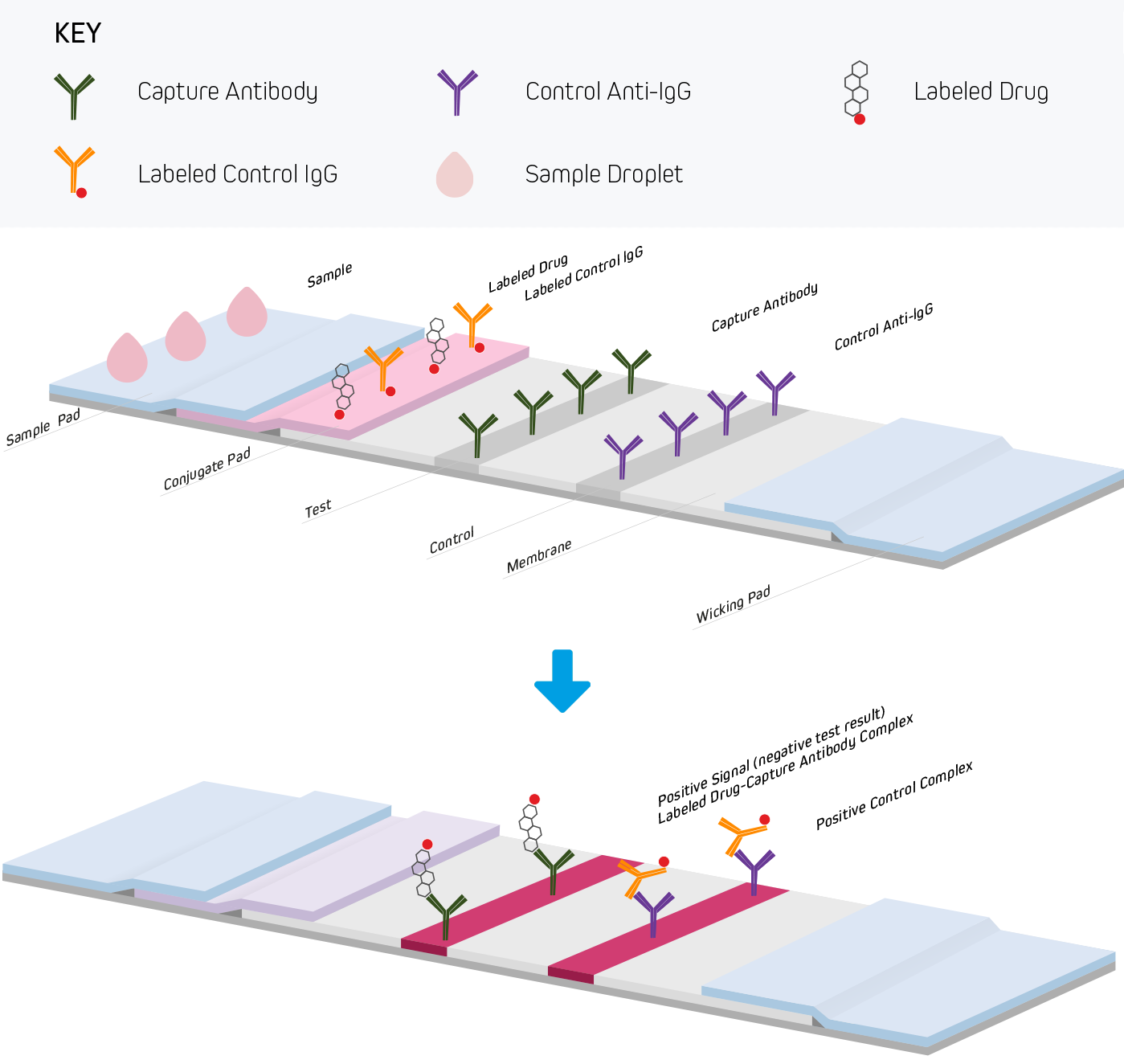
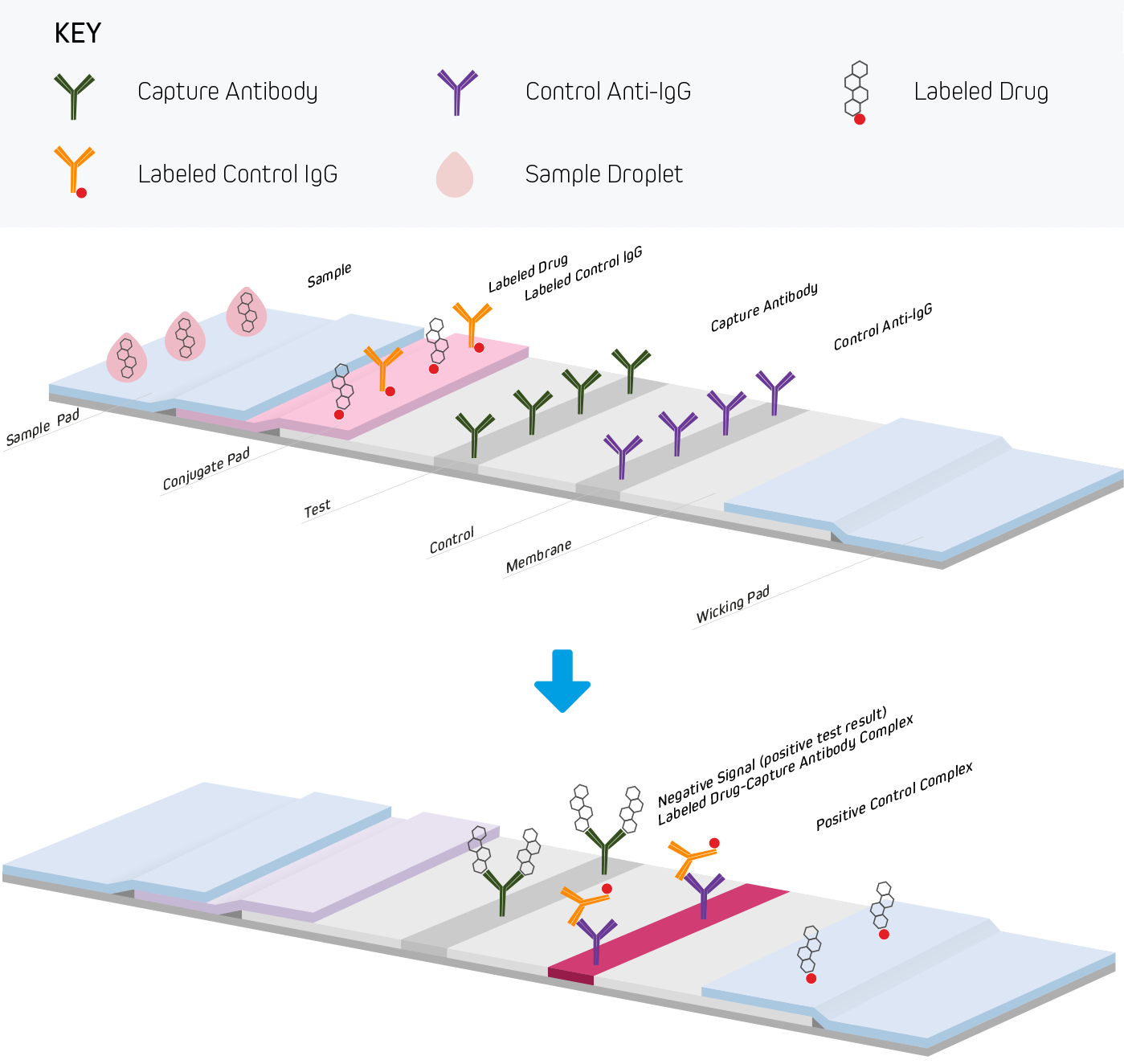
Lateral flow immunoassays are performed in one of three common formats. They include direct detection (Figure 1), sandwich (Figure 2), and competitive (Figure 3) approaches. Each test is run in a linear strip format, made from materials necessary to separate and detect the analytes of interest and provide a control reaction to confirm the test was performed correctly. The following examples outline the basic principles of lateral flow immunoassays and briefly highlight the strip’s components and their functions.


Choosing the correct antibody is a key factor in developing a successful lateral flow immunoassay. “If the antibodies are not meticulously selected for their ability to recognize the target antigen(s) in this format, no amount of optimization will be able to overcome this inherent defect” (Brown, 2008)
Two fundamental properties of an antibody mostly govern LFIA performance, those being affinity and specificity.
Affinity is particularly important because the relative abundance of the antigen may be low. Since antigen, antibody conjugate, and the coated antibody interact for mere seconds in the test strip’s analytical region, fast kon rates and slow koff rates are desirable.
The antibody must recognize the target antigen specifically and not detect similar or homologous proteins and molecules. Because of the high concentrations of antibodies used in LFIA tests, excellent specificity is also essential as most strips are coated with antibodies at concentrations ranging from 1-3 μg per linear cm across the membrane within a small bed volume of approximately 0.1 mm3. This results in antibody concentrations that are typically 25-100 times greater than those coated in ELISA plate wells. Colloidal gold antibody conjugates are often used at 30-250 ng per test. If it is assumed that the leading front of a sample rehydrates the labeled antibody in 10-50 μl, then the antibody conjugate concentration will be between 0.6 and 20 μg/ml. At the higher concentration, the antibody may be 100x its Kd, which could drive non-specific interactions from weakly binding, less-specific antibodies, resulting in false positives.
Read more about cross-adsorbed antibodies.
Antibodies for LFIAs may be made in house or purchased from commercial sources.
One of the first considerations is the quantity required to launch and sustain a commercial product. If one coats 1μg of capture antibody per strip and wishes to make 1 million strips, the amount of antibody required to do so would be a minimum of 1g. Therefore, ensuring that suppliers can manufacture large volumes with consistent quality is paramount. If the antibody is to be manufactured in house, care must be taken to select an appropriate antigen, immunization method, screening strategy, and scale-up process.
Monoclonal antibody (MAbs) development and production is an effective way to acquire antibodies with the desired attributes and the promise of consistent quality. However, scale-up under in vitro conditions can be expensive, and lot to lot variation can arise between purification runs. It is also critical to screen monoclonal antibodies to identify those that perform well when membrane bound, labeled, and alongside other antibodies used in the final assay. It is not uncommon for an antibody to work well as the capture agent when bound to a membrane yet perform poorly when conjugated to a reporter molecule. Finally, screens must also identify antibodies that recognize the epitope in the assay format under experimental conditions, such as buffer composition or conformational structure of the antigen.
Production of polyclonal antibodies (PAbs) is simple to scale-up, whether using rabbits, goats, chickens, or donkeys as host animals. Another advantage to PAbs is that they can be used to achieve greater assay sensitivity as well. Being a mixture of immunoglobulins, each recognizing a different epitope on the antigen simultaneously and in combination, they allow for more reporter molecules to be deposited, increasing signal. PAbs may be subject to lot-to-lot variation however because of their production by the host animal's immune system, which may alter over time.
Validation and quality assurance processes should be used to monitor the specific activity of any antibody, regardless of the source or manufacturing method, to ensure test consistency and reproducibility.
While the development or selection of suitable capture and detection antibodies is critical to the assay’s performance, the choice of control reagents used in the LFIA is also important. Immunoglobulins or secondary antibodies conjugated to reporter molecules are commonly used to create control lines necessary to confirm that a test was performed correctly. For example, positive control lines might be striped with an Anti-Chicken secondary antibody that binds a conjugated Chicken IgY. Chicken IgY is often used because it produces fewer non-specific interactions due to the low homology and sequence identity of the chicken immunoglobulin compared to mammalian immunoglobulins. Control line antibodies should also be minimally cross-reactive to other antibodies used in the LFIA, as control line intensities could vary widely if immunoglobulins used in the test, or present in samples, interfere with the control reagent binding.
Read more about anti-chicken secondary antibodies.
Serological LFIAs use antibodies to capture or detect the immunoglobulins produced by the patient’s immune response. In a serological LFIAs where the test line is coated antigen, and the patient sample contains an anti-antigen immunoglobulin of a particular isotype, signal readout is generated using a conjugated anti-human antibody that complexes with the patient immunoglobulins in the blood that binds to the antigen on the test line (see Figure 1). The specificity of the detection antibody is critical, it should not bind antibodies of other species used in the assay or other isotypes. For example, in an assay detecting IgG and IgM isotypes independently, the anti-IgG antibody must not cross-react to IgM, and the anti-IgM antibody must not detect IgG. Read more about serological testing here.
Reporter molecules commonly used in lateral flow include colloidal gold, latex beads, and fluorescent dyes. Each option has distinct advantages and selection depends on assay goals and detection readout method. Other labels include paramagnetic particles, enzymes, quantum dots, cellulose nanobeads, nano-shells, silica nano-particles, bioluminescent and luminescent materials, and upconverting phosphors (Goryacheva et al., 2013, Quesada-González & Merkoçi, 2015, and (Guo et al., 2021). Read more about reporter enzyme conjugates here.
Colloidal gold is a widely used conjugate for LFIA due to the intense color it generates, ease of conjugation, and the it’s availability with consistent quality. Typical sizes range between 40 and 80 nm, with 40 nm being most common. The intensity of color generated is due to the plasmon absorbance and light scattering of the gold’s electron shell (Huang & El-Sayed, 2010), and the small particles’ ability to pack at high density on the test and control lines.
Conjugation of gold particles to an antibody is usually performed passively via electrostatic and hydrophobic interactions. The two entities are mixed in a low ionic strength buffer, followed by blocking with polyols or proteins like albumin or casein. Colloidal gold is also available with activated surfaces, such as carboxyl groups, allowing for covalent attachment if necessary.
Latex particles are versatile and have been used in lateral flow assays since their initial development. Particles can be loaded with colored and/or fluorescent dyes, paramagnetic particles, and other chemicals. Different dye colors allow assays with multiple simultaneous readouts. Standard sizes used in LFIA range from 100 - 300 μm. Conjugation methods vary between adsorption or covalent linking depending on the surface chemistry. Latex beads may be less sensitive than Gold because their large size prevents them from packing densely at the test or control lines making it is important to consider the choice of incorporated dye.
Fluorescent dyes have found favor as labels; being small molecules (<1 nm), they release more readily from the conjugate pad than larger nanoparticles. Dyes facilitate quantification and detection of multiple analytes by one LFIA device (Wild, 2016), though they require the use of special reading devices to show results and take full advantage of the dye properties. Fluorophores suitable for LFIAs should be economical, have long Stokes shifts, good quantum yields, and be photo-stable. Dyes are available in forms that facilitate covalent conjugation to primary amines and sulfhydryl groups on proteins and small molecules. Fluorescein and Cyanine dyes are popular choices.
Some dyes may not be suitable for LFIA due to photobleaching or quenching at high concentrations. Binding activity may be compromised by covalent conjugation occuring at critical points on antibody paratope or target epitope, or aggregation caused by hydrophobic dyes.
The success of any LFIA also depends on its material components, (Figure 4), and manufacturers commonly use different proprietary methods and reagents for their production. For this reason, evaluation of materials from various sources should be done to ensure an accurate and reliable test be developed. Test optimization is an iterative process that may take months to complete. The following section briefly describes each component, and considers their importance in producing an effective LFIA.
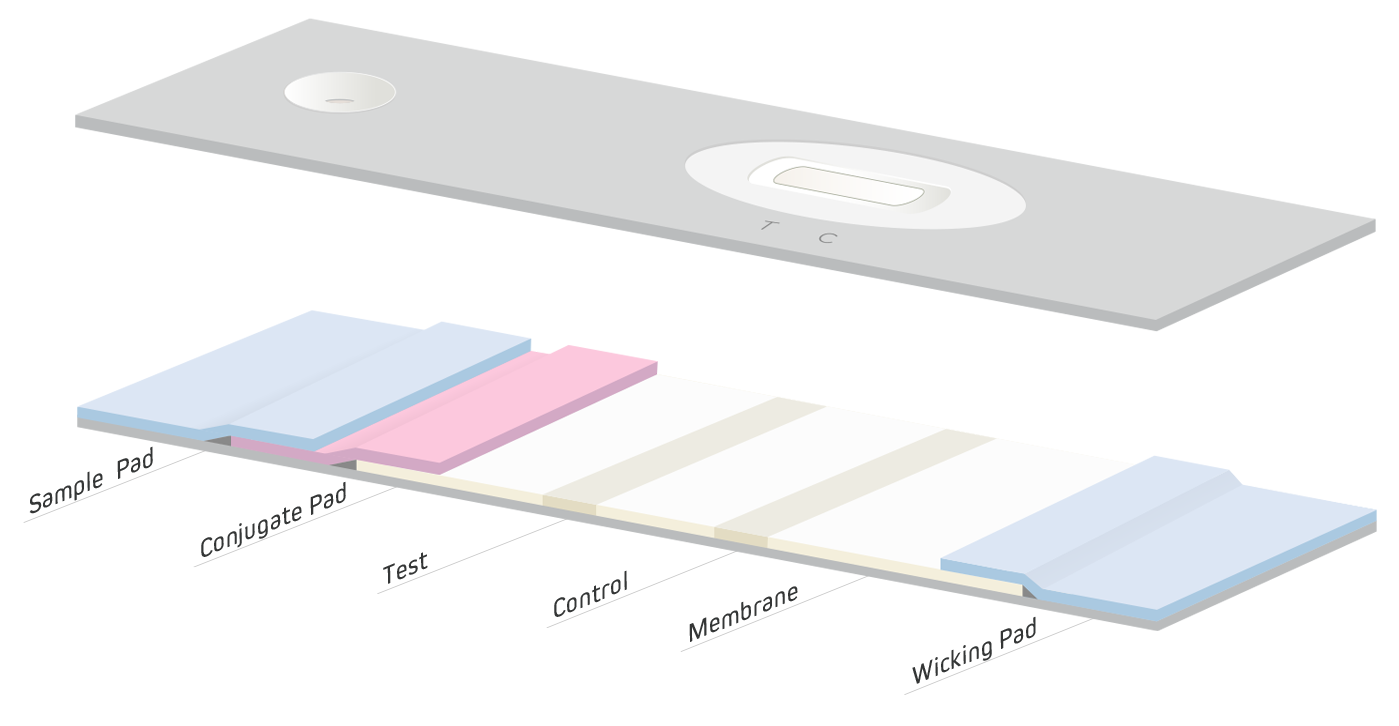

The first step of an LFIA is the introduction of the sample to the device by the sample pad (Figure 4). The choice of pad material depends on the nature of the specimen and analyte being detected.
In samples of blood where the analyte is found in the plasma, it is vital to remove whole blood cells to prevent their interference with the assay which may reduce sensitivity. Cells can be removed using centrifugation, however a sample pad that can filter out cells or biological “debris” expedites the testing process simplifying “Point Of Care” (POC) procedures. The sample pad can also be used to pretreat the sample using chemicals pre-applied to the pad that adjust pH or help block non-specific interactions. Sample pads are typically composed of cellulose or glass fiber.
Free-flowing substances from the sample migrate from the sample pad to the conjugate pad. The conjugate pad acts as a reservoir for antibodies and other proteins or analytes conjugated to reporter molecules, such as colloidal gold, colored latex particles, or fluorescent dyes. Reagents are impregnated into the pad by immersion or by using special liquid dispensers. Additional components, such as sugars, may be added to ensure the dried impregnated reagents’ stability at ambient temperatures, and extend shelf life.
As the sample migrates into the conjugate pad, the conjugates are rehydrated and interact with the sample components. They then travel on to the membrane, which it overlaps slightly. Conjugate pads are typically made from glass fibers, polyesters or rayons.
Reagent-sample mixtures migrate through the membrane where the immunoassay is completed and visualized. The membrane used in an LFIA influences sensitivity, speed and overall background (Huang et al., 2016 and Wild & Mansfield, 2016). Faster flow rates can reduce background, but can compromise sensitivity, resulting in false negatives. Slower wicking rates are exploited when high sensitivity is required because analyte resident time is increased. Slow wicking rates can cause false-positive signals due to higher backgrounds caused by antibodies that are not adequately specific.
Nitrocellulose membranes are commonly used due to their high protein-binding capacity and wide availability. Membrane manufacturers add proprietary surfactants, wetting agents, and other chemicals to control protein binding and wicking rates so consistent assay performance can be achieved.
Antibodies applied to nitrocellulose membranes bind upon contact via hydrophilic interactions. Antibodies bind at the point of application, and do not diffuse with the buffer. After antibody application, water is driven off under forced air at 40°C, and the antibodies are cured onto the membrane by hydrophobic forces.
Membrane blocking, if required, is performed by immersion into a blocking buffer followed by additional drying.
Effective drying is critical to performance of the LFIA as it ensures the stability of the biomolecules and uniform rewetting. Coated materials are stored under carefully controlled temperatures and humidities of around 20%.
A wicking pad at the distal end of the lateral flow strip draws the carrier liquid/buffer from the nitrocellulose membrane, after it passes over the test and control lines, to the end of the strip. Sample and conjugate continue to be drawn through the test strip until no more liquid can be wicked, or the wicking pad becomes saturated. High-density cellulose is often used for this purpose.
To ensure an LFIA behaves in a predictable and reproducible manner, antibodies, antigen, buffer salts and conjugates must be applied to the membranes or pads in a uniform and reproducible manner. A variety of methods can be used depending on the material being coated, or phase of the development or manufacturing process. LFIA manufacturing requires equipment that can produce consistent flow characteristics and application methods that can achieve high throughput.
Bulk materials such as buffers, blocking agents, and sometimes conjugates are impregnated by immersion of the pad into solutions containing these components followed by blotting and drying.
The application of antibodies and proteins to membranes or conjugate pads requires greater precision to obtain uniformity between production lots; therefore, specialized dispensing equipment is necessary.
Pneumatically driven contact tip dispensers apply liquids by pumping material through flexible tips placed in contact with a membrane, or conjugate pad. Either the dispensing tip or membrane moves in reference to the other to create a uniform line of reagent along the material.
Contact dispensers are often economical and can be accommodated in a small space. They can damage membranes however, and may be difficult to control in large scale manufacturing operations. Consequently, they are usually used during research and development, where low volumes of strips are required for assay optimization.
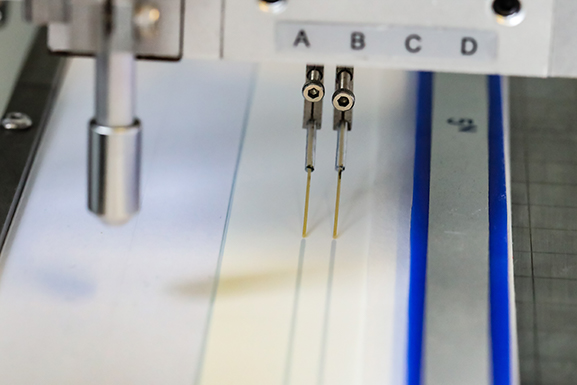
Contactless dispensing devices, such as airbrush or inkjet droplet dispensers, utilize high precision pumps to deliver down to nL-sized drops that can create lines/stripes as small as 200 μm wide. Forced air can also be introduced into the flow to dispense a spray similar to an artist's airbrush.
Both non-contact methods can deliver reagents with CVs of, or better than, +/- 1% appropriate for large scale manufacturing.
The material components of an LFIA are laminated onto an adhesive coated flexible plastic backing to provide rigidity so the test strip can be handled easily. Sufficient overlap at each materials’ interface, minimally 2 mm, is necessary, so the sample liquid flows seamlessly from one section to another. When the materials are applied to the backing, uniform pressure is used to ensure the sample runs evenly along the test strip. After this “card” is assembled, strips are cut in consistent widths of approximately 5 mm. Strips can then be placed into carefully designed and molded plastic housings to ensure the end user applies the sample in the correct area and that proper flow is achieved. A test window with appropriate markings is also included in the plastic housing to facilitate correct result read out and/or validity of the test.
Test strips need not be assembled with sample or conjugate pads. Strips constructed in this fashion are often referred to as “half strips” or “dip sticks”. They are commonly used during development to screen antibodies, conjugates and other reagents. A small amount of sample and reporter conjugate, 50 μl for example, are mixed in a test tube or 96 well plate, and the free membrane end of the half strip is placed into the solution. The mixture migrates up the strip by capillary action and eventually reaches the wicking pad. The strip can be removed and read, or transferred to tubes containing other solutions that are drawn up until the wicking pad is saturated.
Having provided this introduction to Lateral flow immunoassay formats and construction, an example of the development of a simple test to individually detect human IgG and IgM is presented in the following section.
Serological tests enable disease surveillance from the initial infection through to the development of immunity. The power of serological testing comes from the specific detection of patient antibodies generated by the immune system. Lateral flow immunoassays are widely used to detect human immunoglobulins. Here we demonstrate the utility of Anti-Human isotype specific antibodies from Jackson ImmunoResearch in the LFIA format.
A sandwich assay based half strip lateral flow experiment was constructed to specifically detect human IgG vs IgM in human serum. Control lines were included to show the test was performed correctly. The figure below represents the assay setup and interactions indicative of a positive test.
Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Ms, Rb Sr Prot) (109-005-170)
40 nm gold labeled Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Ms, Rb Sr Prt) (109-405-170) Specific to the IgG isotype binding crystallizable domain of IgG, with minimal cross reactivity to human IgM.
Goat Anti-Human IgM, Fc5μ fragment specific (min X Bov Sr Prot) (109-005-129)
40 nm gold labeled Goat Anti-Human IgM, Fc5μ fragment specific (min X Bov Sr Prot) (109-405-129). This antibody is also isotype specific and is produced to have minimal binding to IgG. (109-005-170)
Donkey Anti-Chicken IgY (IgG) (H+L) (min X Bov, Gt, GP, Sy Hms, Hrs, Hu, Ms, Rb, Rat, Shp Sr Prot) (709-005-155)
40 nm gold labeled ChromPure Chicken IgY (003-400-003).
Strips were blocked with Bovine Serum Albumin IgG-Free, protease-Free (001-000-161).
Antibodies were applied to Whatman FF170HP nitrocellulose membrane in lines to produce strips that would mirror typical LFIA flow characteristics. Capture antibodies were diluted to at 0.3 mg/ml in PBS and continuously dispensed at 60 μl/min onto a membrane moving at 10mm/s. Dispensing speed was controlled by use of a syringe pump through PEEK tubing in direct contact with the membrane.
Following striping, the membrane was dried at 40°C under forced air, after which the membrane was blocked by immersion into a solution of 1% BSA in PBS for 1 hour, followed by washing and then drying overnight at 40°C under forced air.
After drying, the membrane was applied to a self adhesive backer card and a cellulose wicking pad (Ahlstrom Grade 222) overlapping the nitrocellulose membrane by approximately 2mm was added. The membrane was cut to produce individual strips of 0.5 cm which were placed in a desiccator containing drierite for a minimum of 48 hours before use.
Nitrocellulose membranes were striped with capture antibodies resulting in lines containing 0.3 µg of antibody per linear cm: IgG test line: Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Ms, Rb Sr Prot) (109-005-170), IgM test line: Goat Anti-Human IgM, Fc5μ fragment specific (min X Bov Sr Prot) (109-005-129) and Control test line: Donkey Anti-Chicken IgY (IgG) (H+L) (min X Bov, Gt, GP, Sy Hms, Hrs, Hu, Ms, Rb, Rat, Shp Sr Prot) (709-005-155). Membranes were blocked with 1% Bovine Serum Albumin (IgG-Free, Protease-Free) (001-000-162) in PBS followed by drying and the application of a wicking pad.
The Lateral flow test strips were placed into 50 µl mixtures containing detection antibodies: 40 nm gold conjugates, at 0.8 OD520/ml of ChromPure Chicken IgY (IgG)-whole molecule (003-400-003), Goat Anti-Human IgM, Fc5μ fragment specific (min X Bov Sr Prot) (109-405-129) and Goat Anti-Human IgG, Fcγ fragment specific (min X Bov, Hrs, Ms Sr Prot) (109-405-098).
Additional wells were also spiked with 50 ng of purified human immunoglobulins: ChromPure Human IgG, whole molecule (009-000-003) and ChromPure Human IgM (myeloma), whole molecule (009-000-012) or serum human serum diluted 1:200 in PBS. After running the test for 15 minutes, the dipsticks were transferred to wells containing PBS to remove excess gold conjugate.
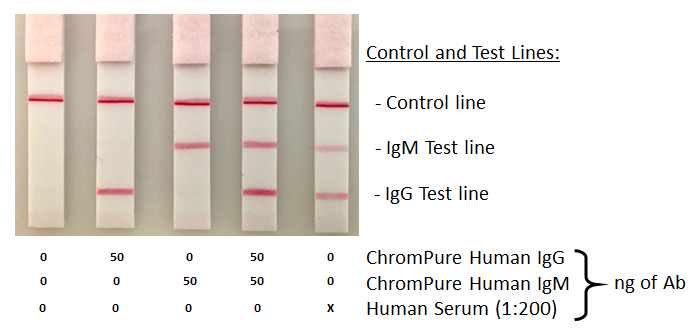
All tests were performed in wells of a 96 well microtiter plate and took less than 20 minutes to complete. Reagent concentrations and other parameters required optimization. These included, capture antibody, detection conjugate, assay buffer conditions, and need to block the membrane. Final conditions are detailed in the method summary.
Capture and detection antibodies generated strong positive test lines indicating detection of the specific immunoglobulin isotypes in presence of human serum. While not shown here, sub-nanogram quantities of human immunoglobulins were also detected in this format.
The use of neat human serum did generate a “hook effect” however resulting in loss of signal the more concentrated the sample was in serum Thus, it was essential to dilute the serum 1:200 in PBS before application. Dilution can be avoided by using larger quantities of gold conjugate, but this may lead to difficulty in result interpretation and require a chase buffer wash step to aid analysis. A membrane wash step was included in this experiment to improve clarity.
Blocking with BSA and buffering under physiological conditions with Tween 20 improved sample flow. The cellulose wicking pad was highly effective, enabling the entire sample to be drawn up if left for extended periods of time.
This brief case study demonstrates JIR antibodies are suitable for the detection of specific immunoglobulin isotypes from human serum, demonstrating their suitability for testing in conditions required for LFIAs used at point of care (POC) testing.