
"I have used a wide variety of secondaries and Jackson ImmunoResearch has consistently been the best. The fluorophores are bright and stable and their selective (x reactivity removed) secondaries have always shown species specificity in multiple labeling."
Janet Duerr, Ohio UniversityRating: 5.0
Green Fluorescent Protein (GFP) is a common protein tag used to generate fusion proteins that may be expressed in prokaryotic and eukaryotic systems. The GFP tag is a useful reporter molecule because it does not require exogenous substrates or cofactors to generate fluorescence. GFP is employed in numerous applications, including quantification of gene expression, protein localization within living organisms, studying protein interactions, and as a biosensor. The addition of an Anti-GFP antibody can often improve experimental performance and overcome limitations that use of the tag itself may incur.
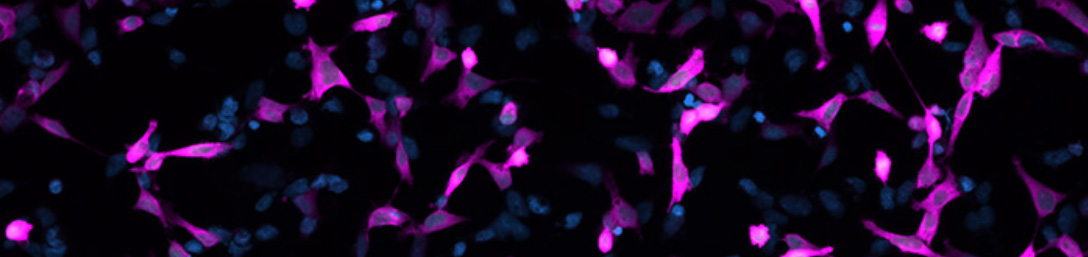
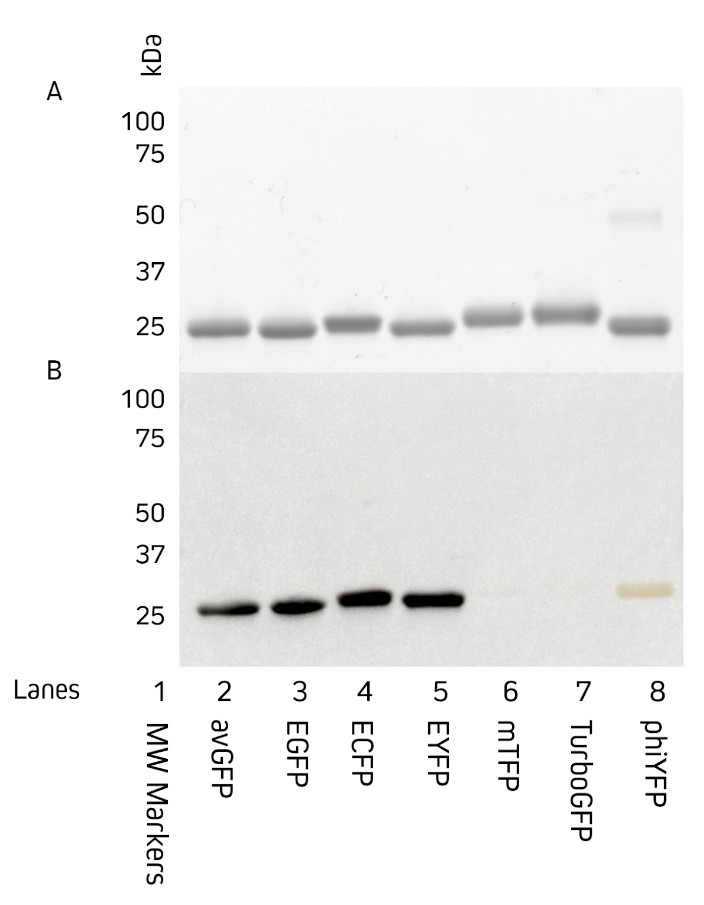
Jackson ImmunoResearch AffiniPure® Rabbit Anti-GFP is an affinity-purified polyclonal antibody raised in rabbit and can be used to detect Aequorea victoria Green Fluorescent Protein (avGFP) and its derivatives, including EGFP, ECFP, and EYFP (Fig. 1).
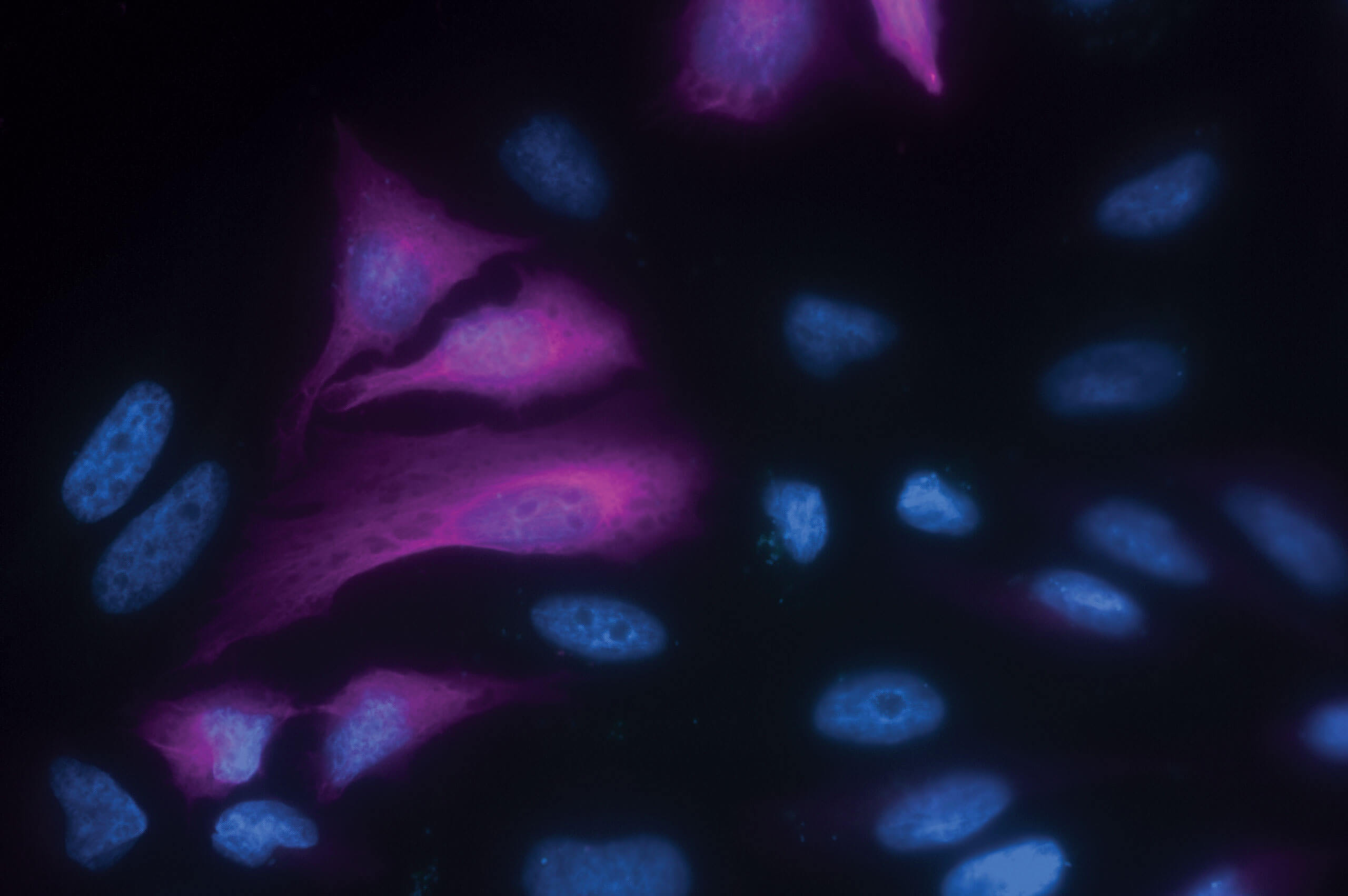
AffiniPure® Rabbit Anti-GFP antibody can be used to amplify the signal from GFP expressed by cells in Immunohistochemistry (IHC) or Immunofluorescence (IF) experiments. Our rabbit polyclonal Anti-GFP antibody may also be used for immunoprecipitation (IP), ELISA, ICC, Flow cytometry, and Western Blotting.
Jackson ImmunoResearch AffiniPure® Rabbit Anti-GFP antibody is available conjugated to a range of reporter molecules. For fluorescence imaging, Jackson ImmunoResearch Rabbit Anti-GFP antibodies conjugated to Alexa Fluor® dyes can deliver bright target signal and spectacular signal amplification.
Anti-GFP antibody conjugates can add versatility to an assay when trying to work around autofluorescence or equipment limitations, such as when a laser line or channel is occupied by another target. Using a fluorophore that emits in a different channel, Anti-GFP antibodies can circumvent these limitations, making them useful for detecting multiple targets in the same experiment.
| Conjugate | Excitation Peak (nm) | Emission Peak (nm) |
|---|---|---|
| Alexa Fluor® 488 | 493 | 519 |
| Alexa Fluor® 555 | 552 | 572 |
| R-Phycoerythrin, R-PE | many, 488 | 580 |
| Alexa Fluor® 568 | 577 | 602 |
| Alexa Fluor® 594 | 591 | 614 |
| Alexa Fluor® 647 | 651 | 667 |
| Alexa Fluor® 680 | 684 | 702 |
| Alexa Fluor® 790 | 792 | 803 |
AffiniPure® Rabbit Anti-GFP conjugates also include popular reporter enzymes, Horseradish peroxidase (HRP), and Alkaline phosphatase, as well as a biotin conjugate facilitating the use of chromogenic IHC staining techniques and amplification protocols such as ABC (Avidin-Biotin-Complex) and LSAB (Labeled streptavidin-biotin). Switching to a non-fluorescent detection system enables the use of traditional light microscopy techniques, such as chromogenic IHC.
| Product Description | Product Code | |
|---|---|---|
| Peroxidase AffiniPure® Rabbit Anti-GFP | 300-035-245 | |
| Alkaline Phosphatase AffiniPure® Rabbit Anti-GFP | 300-055-245 | |
| Biotin-SP (long spacer) AffiniPure® Rabbit Anti-GFP | 300-065-245 |
Western Blot and ELISA (Enzyme-linked immunosorbent assays) can be used to both confirm and quantify protein expression. GFP is a popular reporter molecule used to identify successful protein expression in the absence of protein-specific antibodies. Read our article exploring the use of JIR Anti-GFP antibodies to assess protein expression qualitatively and quantitatively.
Flow cytometry is a useful technique for screening and quantifying protein expression, particularly when they are expressed on the cell surface. The GFP tag is frequently used as a reporter molecule to track protein expression by flow cytometry. Learn how Jackson ImmunoResearch Anti-GFP antibodies can be used to detect a GFP fusion protein expressed on yeast cells by flow cytometry.
Table 2: AffiniPure® Rabbit Anti-GFP antibody conjugates
